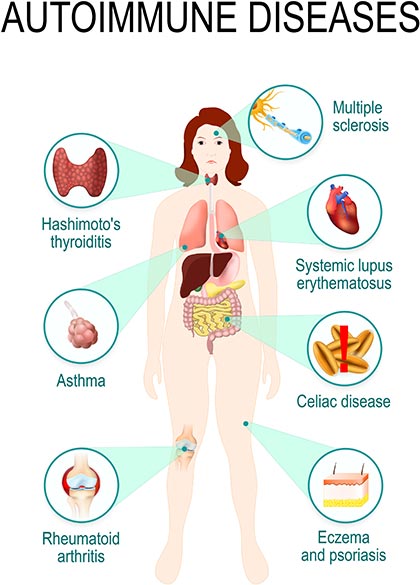
 There are about 80 known autoimmune disorders affecting 5-8 per cent of the population worldwide.1 These range through psoriasis, rheumatoid arthritis and coeliac disease (affecting about one in 100 people), type 1 diabetes, multiple sclerosis and rheumatic heart disease (affecting about one in 1000), and ulcerative colitis and Crohn’s disease (affecting one in 10,000) and more rare diseases.2
There are about 80 known autoimmune disorders affecting 5-8 per cent of the population worldwide.1 These range through psoriasis, rheumatoid arthritis and coeliac disease (affecting about one in 100 people), type 1 diabetes, multiple sclerosis and rheumatic heart disease (affecting about one in 1000), and ulcerative colitis and Crohn’s disease (affecting one in 10,000) and more rare diseases.2
Autoimmune diseases are identified by the presence of autoantibodies, which attack the body’s own cells. Some autoimmune disorders are clearly linked to autoantibodies, others generate autoantibodies but the link between them and the pathology of the disease is unclear. Another group of diseases, such as psoriasis and ulcerative colitis, have no clearly identified autoantibodies but respond well to immune-modifying treatments.2
Autoimmunity occurs where the T- and B-lymphocytes stop distinguishing “self” from “non-self” and begin generating antibodies and immune memory cells against the body’s own tissues. This can cause body-wide effects, such as systemic lupus erythematosus, or target a single organ as with type 1 diabetes.
Despite considerable understanding of the mechanisms and genetics of some autoimmune diseases, our understanding of the triggers for these diseases and their subsequent development is poor.1 Study of the human genome has identified genes that predict inherited autoimmune risk. The greatest predictor of severe disease is found in HLA genes that code for the human leukocyte antigen – a molecule on the surface of all body cells that helps to identify them as “self”.1 Other genetic screens have identified other more disease-specific variant genes coding for different molecules. As further detail of these variant types emerges, they could potentially be treated through gene-editing technologies such as CRISPR.
Environmental factors
Environmental factors (such as smoking, obesity, diet and infection) are known to increase risk, but the mechanisms by which they cause autoimmune disease are not understood. It is thought that they may directly damage or alter the immune system but also cause damage to body tissues that further trigger autoimmune responses and delay healing – setting up a cycle of inflammation, tissue damage and immune attack.1
Studies have shown a link between some autoimmune disorders and the microbiome of the gut and/or skin. This may be due to changes in, or abnormal, microbiota, escape of bacteria from the gut into the body, or cross-talk between certain species of microbiota and the person’s immune system. Dietary intake may affect risk of autoimmune disease via its effects on the microbiota. For example, fasting and calorie restriction in animal models affect immune cell types and their distribution in the body, reducing risk of autoimmune disorders.1
The past two decades have seen huge gains in the management of autoimmune disease through the development of monoclonal antibodies…
Current treatments for autoimmune disease involve non-specific immune-modulating drugs with limited efficacy and high risk of side effects due to immune suppression (eg infections, malignancies).1 Generally, patients begin therapy with disease-modulating drugs such as methotrexate (for rheumatoid arthritis) or corticosteroids. The past two decades have seen huge gains in the management of autoimmune disease through the development of monoclonal antibodies – drugs that target signalling molecules in the immune pathway – eg infliximab and tocilizumab. However, these new therapies are also non-specific and have system-wide side effects.
There is a growing body of research directed toward therapies that target individual autoimmune conditions, are patient-centric and that address underlying causes.1
Ultimately, the goal for immunologists is the ability to accurately predict risk of autoimmune disease and prevent or delay its onset. This has already been seen in a small study where a monoclonal antibody against a type of T-cell that attacks insulin-producing pancreatic beta cells in susceptible people, prevented or delayed by an average of two years the onset of type 1 diabetes.3
References
- Fugger, L., Jensen, L., & Rossjohn, J. (2020). Challenges, progress and prospects of developing therapies to treat autoimmune diseases. Cell, 181(1), 63-80.
- Bender, M., Christiansen, J., & Quick, M. (2021). Autoimmune Disease, by the Numbers. Scientific American 325(3), 31-33. doi:10.1038/scientificamerican0921-31
- Herold, K., Bundy, B., Long, S. A., Bluestone, J., DiMeglio, L., Dufort, M., Gitelman, S., Gottlieb, P., Krischer, J., Linsley, P., Marks, J., Moore, W., Moran, A., Rodriquez, H., Russell, W., Schatz, D., Skyler, J., Tsalikian, E., Wherrett, D., Ziegler, A.-G., Greenbaum, C., & Type 1 Diabetes TrialNet Study Group. (2019). An anti-CD3 antibody, Teplizumab, in relatives at risk for type 1 diabetes. New England Journal of Medicine. 381, 603-613.




