Despite decades of research into the effects of diet on cardiovascular disease (CVD), debate continues about what is the optimum cardio-protective diet. The result is uncertainty for nurses and patients as they struggle to make sense of the often-contradictory statements in the media.
This article presents the widely accepted research findings and seeks to clarify the more puzzling results, providing a way forward for nurses wishing to conduct an informed discussion with patients.
It also presents an educational tool/table that can be used for clients with good health literacy and for staff education purposes. The article also provides useful comparisons of the benefits and risks associated with a range of popular diets.
Introduction

Many nurses routinely provide lifestyle education, especially after checking clients’ lipid profiles; but the dietary advice given is often generic, rather than targeted to their individual cholesterol results.
There is considerable debate in the scientific and lay media about the role of different foods and serum cholesterol levels in the development of CVD and whether it is possible to achieve meaningful change in serum lipids through dietary changes alone.1, 2
Sceptical clients may prefer alternative diets to the traditional “low cholesterol” diet, such as low carbohydrate (including Atkins and keto), low glycaemic index (GI), or the Mediterranean diet. Nurses should be able to discuss the relative merits of different dietary regimens with their patients.
This article presents an educational tool that identifies the effects of different foods and dietary patterns on serum lipids and overall cardiovascular risk. The tool was first published in 2006,3 and updated soon after, following feedback from clinicians using it.

Knowledge in this research area has progressed significantly in the intervening years, so the tool and article have now been updated. It can be used to give targeted advice on how to improve specific serum lipids and overall cardiovascular risk. It is suitable to educate health professionals and clients with moderate health literacy.
Current research supports the benefits of nurses providing dietary and lifestyle education to clients, especially those with established cardiovascular disease, in a variety of practice settings.4, 5
The tool is available to download at the end of the article. Hopefully, readers will adopt it in their workplace and report their experiences and recommendations to the lead author. Future research is planned into the utility of the table as a teaching tool.
Sources of research evidence
Historically, evidence for effects of food on cholesterol profile have tended to come from short-term controlled feeding studies, while the effects of lifestyle on morbidity and mortality have been inferred from large-scale, long-term, observational studies. This has meant a causal relationship between diet, cholesterol and heart disease is largely inferred, rather than proven.
Current research supports the benefits of nurses providing dietary and lifestyle education to clients, especially those with established cardiovascular disease.
More recently, intervention studies have followed people on a prescribed diet for years, (for example the Mediterranean diet) and monitored their health outcomes (such as CVD morbidity and mortality), seeking the all-important causal relationship.
Meta-analyses have combined data from multiple similar studies to increase their power and provide more compelling results. However, this process comes with its own challenges due to interpretation and management of differences between studies.
Other issues with such long-term intervention studies are confounding variables, because when someone follows a “healthy diet”, they are also likely to exercise regularly and seek regular medical attention.1
Despite the increasing volume and quality of the evidence in lifestyle research, some questions and debates remain, especially related to dietary fat consumption, the main points of which will be discussed in this article.
The evidence used here is predominantly from current meta-analyses and systematic reviews of intervention studies and observational studies. This article aims to provide a balanced and useful educational resource to aid nursing practice.
Background
Atherosclerotic cardiovascular disease (CVD) includes coronary heart disease (CHD), cerebrovascular disease, and peripheral artery disease.6 Other conditions will be identified that also benefit from the dietary changes being discussed, such as type two diabetes (T2D) and cancer.
The lipid profile and its components: total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides, help determine an individual’s CVD event risk.
Lipids, principally cholesterol and triglycerides, are water-insoluble compounds that require larger protein-containing complexes called lipoproteins to transport them in blood. Of these lipoproteins, low density and very low-density lipoproteins predispose to atherosclerotic arterial plaques, while high density lipoproteins offer a degree of protection against atherosclerosis.7
Lipid testing
A fasting lipid blood test will provide a client’s LDL, HDL and total cholesterol levels, plus their total/HDL cholesterol risk ratio and triglyceride level. The ratio of LDL to HDL is important in determining CVD risk. This ratio is increased when the level of LDL, the “bad” cholesterol, is too high or the level of HDL, the “good” cholesterol, is too low.7
Triglycerides contain fatty acids and are transported by atherogenic very low-density lipoprotein (VLDL), hence raised levels of triglycerides are also associated with an increased risk of ischaemic heart disease.7 The results of the fasting lipid blood test can be used to determine which components of the cholesterol profile require modification to reduce cardiovascular event risk.
Introducing the tool
CARDIOVASCULAR RISK FACTOR MANAGEMENT TOOL
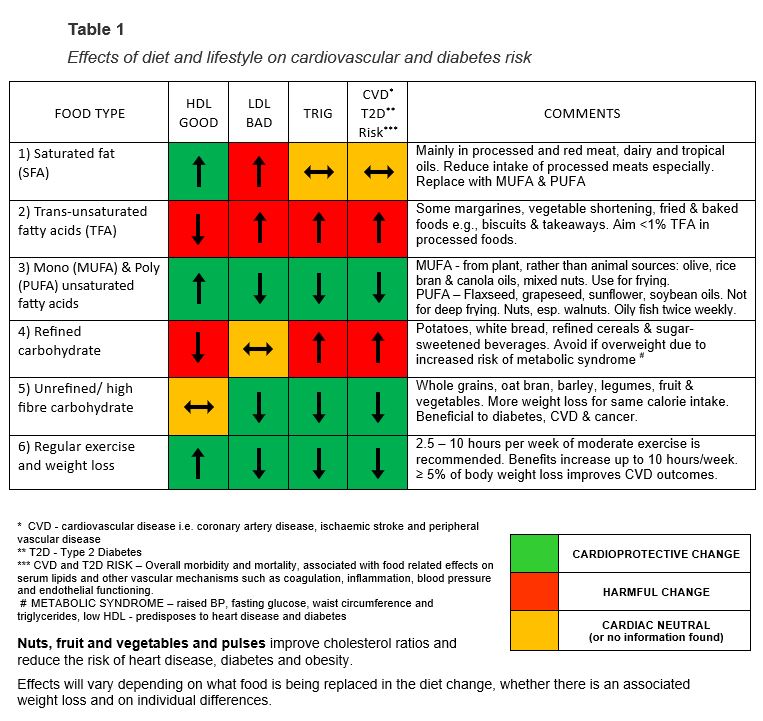
(NB: this tool is available as a downloadable PDF at the bottom of this article.)
In the tool (Table 1, above), the first three columns relate to the different components of the client’s lipid blood test (HDL, LDL and triglycerides), to determine which dietary and lifestyle changes would improve their cholesterol. The fourth column identifies how dietary changes could improve clients’ overall CVD and T2D risk.
It is now recognised that the relationship between diet and CVD morbidity and mortality is more complex than changes in cholesterol/lipid profile alone. For example, diet can also improve vascular endothelial and clotting function, while reducing insulin resistance and inflammatory markers, all of which have been demonstrated to reduce CVD and diabetes risk.1 It was important to demonstrate changes to these non-cholesterol risk factors on the table as well.
Navigating the tool
The columns intersect with rows to identify the effects of various dietary and lifestyle changes. Rows one to three show specific fats, rows four to five compare refined and unrefined carbohydrates, and row six shows other beneficial lifestyle changes.
Each box contains an arrow, indicating the direction of the change to the cholesterol or risk value. A traffic light system highlights the results of beneficial dietary changes in green, neutral changes in yellow, and harmful changes in red.
There are two ways of navigating the tool:
- Working with an individual patient, it is often best to follow columns based on the aspects of the patient’s cholesterol profile that need to change.
- In a group teaching session, it is often better to follow the rows which show how certain dietary and lifestyle changes will affect CVD risk.
Using the tool
For example, if a client has a low HDL, following the first column indicates this could be increased by weight loss, exercise, and an increased intake of monounsaturated and polyunsaturated fats.
Foods that should be reduced include trans-unsaturated fatty acids, such as baked and fried foods, and refined carbohydrates, such as processed/packaged foods and white bread.
Saturated fatty acids: Although the first column shows saturated fatty acids (SFAs) increase HDL, a positive change, row one shows they also increase LDL, a negative change, leaving overall CVD risk unchanged.
By comparison, increasing polyunsaturated fatty acids (PUFA) or monounsaturated fatty acids (MUFA) instead, can reduce CVD event risk by 17-21 per cent. The greater the SFA reduction, the greater the improvement in CVD risk (row three).2, 8
The role of saturated fats in the development of CVD remains controversial, as they are a very diverse food group, with varying effects on observed CVD and diabetes risk.
Hence, increased intake of SFA, especially red and processed meat and tropical oils, is not recommended here.6, 9 This highlights the importance of checking along the relevant table row for possible harmful effects before recommending a dietary change.

The role of saturated fats in the development of CVD remains controversial, as they are a very diverse food group, with varying effects on observed CVD and diabetes risk.8 For example, intake of processed meat like ham, bacon, salami increases CVD risk and is harmful, also due to high sodium content, so should be discouraged.
But some foods containing SFA are potentially beneficial, such as dairy (especially yoghurt), nuts and vegetable oils.10, 11, 12
Trans-unsaturated fatty acids (TFA): The effect of various dietary changes on the lipid profile, such as increasing the intake of certain fats, or replacing saturated fats with carbohydrates, can be determined by following the appropriate row of the tool.
Row 2 emphatically illustrates how harmful high trans-unsaturated fatty acid (TFA) intake is. It is associated with a 20 per cent increased risk of CVD mortality.13 Some TFAs are produced when a liquid oil is hydrogenated to make it firmer, for use in food production. Many TFAs are unidentified in biscuits, crackers, packaged snacks, and commercially prepared fried foods.1
There is no labelling requirement to identify TFA content in food in New Zealand, unless the manufacturer makes nutritional claims about fat content.14 Looking for foods with less than 1 per cent TFA is recommended, as is preparing food at home.8
Poly-unsaturated fatty acids (PUFA): Replacing either refined carbohydrates or SFA with polyunsaturated fatty acids (PUFAs) is recommended,8 and is shown as a fully positive change in row three, with some improvement in all components of the CVD risk.8

Although there have been some variable cholesterol results in intervention studies,10 they are predominantly positive.2, 13, 15 Also, there is persuasive evidence from both intervention and observational studies of their reducing the incidence of coronary heart disease, myocardial infarction, and arrhythmia.2, 8, 15, 16
The main PUFAs are omega 6 and 3 fatty acids. Omega 6 comes mainly from plant oils such as soybean, safflower and sunflower, and benefits cholesterol and glycaemic control.8 Omega 3 can come from plants such as nuts, especially walnuts and from fish sources, especially cold-water oily fish such as salmon, anchovies and tuna, which are recommended once or twice weekly.8, 17
Mono-unsaturated fatty acids: The value of mono-unsaturated fats (MUFAs) in the prevention of CVD is also contested by some.10, 18 But on closer inspection, the nature of MUFAs is significant in explaining differences in research results.
Those coming from plant, rather than animal origin, are particularly beneficial, especially in the Mediterranean diet (olive oil, avocadoes, and nuts). Animal sources include red meat and dairy and are less beneficial.19
Row 3 of the table shows MUFAs as improving all aspects of the cholesterol profile and CVD risk,20 therefore being a recommended alternative to SFA and refined carbohydrates. But it is important to emphasise the benefits of consuming MUFA of plant origin, as those from animal origin can worsen CVD outcomes.8, 19, 21
Refined and unrefined/high fibre carbohydrates: The effects of refined carbohydrates are compared with high fibre/unrefined carbohydrates in rows 4 and 5. Refined carbohydrates are a major constituent of packaged convenience and take-away foods. They are energy-dense and easily consumed. Processing whole grains into white flour increases calorific content by 10 per cent, reduces dietary fibre by 70-80 per cent, reduces vitamins and minerals by 70 per cent and reduces protein content by 25 per cent.22, 23
Substituting carbohydrates for saturated fat not only does not reduce risk, but may increase it and lead to weight gain, if the carbohydrates are refined, or have added sugar.8, 24
For example, one meta-analysis demonstrated that when saturated fat is replaced with refined starches and sugars, there is a 10 per cent increased risk of coronary heart disease (CHD), whereas replacing it with whole grains is linked with a 9 per cent reduction in risk.25 This is an important point, which is often not passed on to patients when suggesting dietary changes.
By comparison, the benefits of increasing fibre are also shown in row 5. Fibre refers to non-digestible components of the plant-cell wall. It reduces LDL and triglycerides and usually leaves HDL unchanged, thus improving the risk ratio.26, 27
Health benefits include decreased blood pressure and inflammatory markers, also reduced risk of CVD, cancer, stroke, T2D and all-cause mortality. Significantly, there is a dose response relationship — the more fibre consumed, the greater the reduction in CVD risk.27, 28

Alcohol: Following evaluation of the initial tool, some practitioners requested that alcohol be included in this updated tool. Some meta-analyses have identified a potential benefit for low to moderate intake of alcohol on serum lipids, CVD risk factors and all-cause mortality,29, 30, 31 but others have been less compelling or have challenged the findings32, 33, 34 and there are concerns about influential vested interests in trial funding.35
Higher alcohol intake raises triglycerides so should be actively discouraged in hypertriglyceridemia.36 For most age groups, any potential CVD benefit would be negated by increased all-cause mortality, such as trauma and breast cancer, if alcohol intake were to increase.37, 38
Any benefits were also quickly reversed, if alcohol intake increased above recommended levels, with increased triglycerides and LDL cholesterol and increased risks of hypertension, heart failure, cardiomyopathy and arrhythmias.37, 39 It is probably unwise to encourage alcohol intake as a preventive measure for CVD,37 so alcohol has not been included in the revised tool.
Red wine contains phenols, tannins, and the antioxidant properties of resveratrol, which are associated with reduced CVD risk.40 Natural resveratrol is also available in grapes, dark chocolate, apples, peanuts, soy, blueberries, cranberries and Itadori tea. However, it is not possible to consume therapeutically meaningful doses solely through red wine or any other food source.41
The health promotion directorate of Te Whatu Ora’s current recommendations42 remain the soundest advice to give patients who wish to consume alcohol safely.
Regular exercise and weight loss: Regular exercise improves all aspects of CVD risk being considered here. It leads to increases in HDL, lowers triglycerides, and often also lowers LDL and blood pressure (row 6).1, 6 Morbidity and mortality progressively improve, as hours spent in moderate exercise increase, between 2.5 and 10 hours per week.43 Row 6 also shows weight loss benefits all aspects of CVD risk, as does maintaining a healthy weight.44

Nuts, pulses and red yeast rice: A high intake of nuts — especially walnuts, almonds, hazelnuts and pistachios — improves all aspects of the cholesterol profile and reduces CVD, diabetes, cancer, obesity and sudden death risk, and is not associated with weight gain.6, 45, 46 The National Heart Foundation recommends three to four small handfuls of nuts or seeds a week (15g per day).9
In those unable to take statins, diets high in dietary pulses reduce LDL,4 including low fat or non-oil dry seeds of leguminous plants such as beans, peas, chickpeas and lentils, which are different from leguminous high-fat oil seeds such as soy or peanuts.47 Red yeast rice has also been shown to reduce LDL in trials, but further research is required, and dosages of proprietary products may vary.1
Dietary patterns
Despite benefits and harms for certain macronutrients — fats, proteins and carbohydrates — it is generally more helpful to consider “eating patterns” rather than specific foods.8, 17 The following section explores the effects of common diets on CVD risk factors.
The diets considered include low-fat, low-carbohydrate, very low carbohydrate (keto and Atkins), low and high glycaemic index (GI), high protein, Mediterranean, vegetarian, and intermittent fasting (5:2 diet).
In the early 2000s, dietary advice was illustrated by the food pyramid, with all fats at the top in the “eat sparingly” section, along with sugary sweets and salt. Bread, rice, pasta and potatoes were at the bottom, with at least six servings a day recommended, and no distinction being made between “refined” and “unrefined” forms of carbohydrates.48, 49
Low fat diet: We now know the total amount of fat consumed is not as important as the type of fat. Therefore, TFA should be avoided and SFA should be at least partially replaced with PUFA and MUFA, rather than reducing the overall amount of fat consumed,9, 50 or replacing it with refined carbohydrates, which don’t reduce cardiovascular risk and may lead to weight gain.25, 50
So, it is important to emphasise that if replacing TFAs and SFAs with carbohydrates, they should be whole grains and fibre-rich carbohydrates, rather than refined ones, because these can be as detrimental to the cholesterol profile as unhealthy fats.
But fat-reduction recommendations have spawned a ‘low-fat’ food industry, that unfortunately, often replaces fat with sugar and has potentially contributed to increasing obesity, CVD and T2D.
A low-fat dietary pattern with healthy carbohydrates like fruits, vegetables, and whole grains, is not associated with weight gain, and reduces CVD risk.28, 51 But fat-reduction recommendations have spawned a “low-fat” food industry, that unfortunately, often replaces fat with sugar and has potentially contributed to increasing obesity, CVD and T2D.22, 50 So it is important to advise clients to check the sugar content in “low-fat” foods.
Low and high glycaemic index diets: The high sugar content in many low-fat foods, combined with increasing refinement of carbohydrates, contributes to the rising glycaemic index (GI) of foods. High GI foods are digested, absorbed, and metabolised quickly, causing a surge in insulin production, as they are more easily broken down by the body, producing peaks and troughs in blood sugar levels and more hunger.52
This process promotes oxidative stress, inflammation, lipid and endothelial dysfunction, insulin resistance and/or metabolic syndrome, all increasing the risk of CVD, diabetes and death.53, 54, 55 Australia and New Zealand recognise the importance of GI, by regulating low GI nutritional content claims on food packaging.56
Low-GI diets are particularly beneficial for those with T2D or pre-diabetes, especially when combined with a high protein or Mediterranean diet.
High-GI diets are high in potatoes, white bread, refined cereals, and sugar-sweetened drinks, while low-GI diets emphasise fruits, vegetables, and whole grains.1 Despite some contradictory research results,27 there is compelling evidence that low-GI diets result in sustained weight and body-fat loss and may produce more satiety and less hunger.52
Low-GI diets are particularly beneficial for those with T2D or pre-diabetes,57 especially when combined with high protein52 or with the Mediterranean dietary pattern, where it may reduce the incidence of diabetes by approximately 20 per cent.58
Low and very low carbohydrate diets: Others have responded to these concerns about low-fat, highly processed carbohydrate diets, by advocating low and very low carbohydrate diets for weight loss, as well as CVD and diabetes management.59, 60, 61
Some report low-carbohydrate diets leading to more weight loss, and a lower percentage of body fat than low-fat diets,51, 62 but one systematic review found no short-term weight loss benefit for low carbohydrate over a balanced carbohydrate diet.63
On a ketogenic, very low carbohydrate diet (such as Atkins and the keto diet), restriction of carbohydrates leads to glycogen mobilisation and ketosis, which promotes rapid weight loss in the short-term. Ketogenic diets may improve all-cause and CVD mortality, but only when consuming higher levels of plant rather than animal proteins and fat.
If more animal-based proteins are consumed — there is a higher risk of cancer and CVD.6, 64 They can also lead to more side effects such as constipation, headache, halitosis, and muscle cramps. It is important to favour healthy fats (MUFA and PUFA) and proteins such as fish, nuts, legumes and poultry, and check cholesterol regularly, when on a low-carbohydrate diet.51, 60, 62
High protein diets: When reducing carbohydrate intake, a higher proportion of a different macronutrient, such as protein, must be consumed. High protein diets have attracted interest recently as a means of losing weight, keeping it off and improving metabolic risk factors for CVD and diabetes.65, 66, 67, 68, 69 Protein appears to promote energy expenditure and a feeling of satiety or “fullness”, while the body composition changes to less fat and more muscle.70, 71
This differs from other diets where both fat mass (FM) and the fat free mass (FFM), especially muscle, are reduced. When muscle is lost, energy expenditure reduces, creating a tendency to regain weight even if calorie intake does not increase.66
This, combined with an increase in appetite, is called “adaptive thermogenesis”,72 and may help to explain why 80 per cent of weight that is lost will be regained within one year.73 This effect can be reduced by a period of weight stabilisation after weight loss.72
It is important to note though, that high-protein diets produce different effects on mortality, dependent on the protein source.
Adaptive thermogenesis is less of a problem on a high-protein diet, especially when combined with exercising, which results in improved muscle power and exercise capacity and protects against muscle loss.74 Also, high protein diets were associated with beneficial changes in the microbiome, that reduced risk factors for diabetes, when compared with a Mediterranean diet.68
It is important to note though, that high-protein diets produce different effects on mortality, dependent on the protein source. Animal proteins, especially from red and processed meats, are associated with higher cardiovascular mortality, while replacing them with protein from plant sources is associated with improved mortality.64, 75
Daily protein consumption up to 1.66g/kg body weight is considered safe, for healthy individuals.65
Mediterranean diet: The Mediterranean dietary pattern has been associated with improved CVD, diabetes, cancer, Parkinson’s and Alzheimer’s disease risks, also CVD and all-cause mortality, in many observational and some intervention studies, especially in secondary prevention45, 76, 77 although results in other intervention studies are not compelling as yet.78
This diet is high in antioxidants and low in TFAs, being high in fruits, vegetables, whole grains, legumes, nuts, and seeds, with low to moderate amounts of fish, poultry, dairy products, and little red meat. Fat comes from olive oil, which is high in MUFA and there is low to moderate wine consumption.1, 78

It is associated with reductions in atherosclerotic disease and improvement in serum lipids and multiple haemostatic factors such as inflammation and insulin resistance.1, 79
Plant-based and vegetarian diets: Plant-based and vegetarian eating patterns may reduce the risk of CVD by 30-40 per cent,80 lower incidence of obesity, T2D and improve lipid profile.81 However, there are many different types of vegetarian diets, ranging from no animal-based foods, vegan – no meat, dairy products, and eggs, to less restrictive forms that allow eggs, dairy products, and some meats.82
They have the potential to be the healthiest diet, but this is highly dependent on the quality of the carbohydrates, proteins and fats being consumed.6 Diets with “unhealthy” plant-based foods, such as refined carbohydrates, are associated with increased mortality.83
More research is required into benefits of various forms of vegetable-based eating patterns,84 but low calcium and vitamin B12 levels are a risk.85
Intermittent fasting: Intermittent fasting (IF) is defined as an eating pattern without calorie restrictions, alternating between periods of fasting and eating.86 It has grown in popularity over the last decade, partially due to the simplicity of this regimen, as between the designated periods of fasting or reduced intake, the dieter is free to eat as desired.87
It can be more effective than calorie restriction for weight reduction88 and can improve lipid profile and diabetes risk factors,89, 90 including improved insulin sensitivity and anti-inflammatory effects.91, 92

Intermittent fasting (IF) is an umbrella term for several different eating patterns. They are classified as intra-week and intra-day intermittent fasting. Forms of intra-week fasting include alternate-day fasting (ADF) and twice-weekly fasting (TWF) (that is, the 5:2 diet, with two days on 500 calories and 5 days on normal eating.93
Intra-day fasting consists of time-restricted eating, for example fasting:eating hours ratios of 14:10, 16:8 (most popular) or 18:6. The six to 10 hours of normal eating happen either earlier or later in the day, depending on personal preference.
Eating earlier in the day and fasting in the evening is metabolically better, but most people prefer to skip breakfast and make dinner their last meal, in which case it is important not to snack after dinner.94
The range of different forms of intermittent fasting create a good potential to individualise the diet to fit personal preference.
The effectiveness varies, depending on the form of IF that is followed, and some patterns are easier to maintain long-term than others. The range of different forms of IF available create a good potential to individualise the diet to fit personal preference.87
Intermittent fasting can be associated with reduced muscle mass, especially in older people.87 The fasting periods stimulate muscle breakdown while the shorter eating periods reduce the opportunities for recovery through consumption of protein rich meals to boost muscle growth.95, 96
Therefore, it is important to undertake resistance training, preferably during a non-fasting period, to avoid this loss of lean muscle mass,97, 98 and to prioritise high-quality protein food sources for muscle protein synthesis.87, 99

This is important advice for clients considering some form of intermittent fasting. If care is taken to preserve lean muscle mass, then IF is at least as effective at reducing fat mass and improving other risk factors for chronic disease as traditional calorie restriction.
Intermittent fasting does not pose more safety risks than other forms of dieting in the general public. However, people with type 2 diabetes should monitor for hypoglycaemia during fasting and it is not recommended for people with an eating disorder.87
Comparing diets
Low fat, vegetarian, low GI, and Mediterranean diets are all associated with health benefits, especially if weight loss is not the primary goal.1, 6 Low-carbohydrate and very low carbohydrate diets may be most effective at short-term weight loss,100 but come with precautions. The satiating effects and other metabolic benefits of high-protein, Mediterranean and low glycaemic index diets appear to improve maintenance of weight loss, irrespective of the initial diet followed.101
The consistent message is the importance of favouring minimally refined, high quality sources of carbohydrates. Also, healthy fats and proteins, ideally from plant sources, are better for CVD risk and better for the planet.102
The following targeted advice is useful for individual patients for CVD prevention:
- for individuals with an elevated LDL, reduce saturated fats and total calories;
- for people with isolated hypertriglyceridemia, a low-carbohydrate diet with fewer calories;
- for the overweight with elevated triglycerides and/or low HDL cholesterol, or pre-diabetes and fatty liver disease, the Mediterranean diet is recommended.103
Maintaining a low to moderate alcohol intake would also be important when triglycerides are raised.1
Dietary change should include reducing refined carbohydrates, processed meat, sodium, TFA and sugar-sweetened drinks, encouraging moderation in unprocessed red meats, poultry, eggs and milk and an increased intake of vegetables, fruits, nuts, fish, vegetable oils, minimally processed whole grains, legumes and yoghurt.1, 6
Current Heart Foundation diet recommendations are consistent with these dietary changes, especially favouring low-GI foods, high intake of fruits and vegetables and replacing saturated and trans-unsaturated fatty acids with mono and poly unstaturated fats.104
Whichever diet a client follows, is less important than their ability to maintain it.
All the diets discussed above potentially improve CVD risk and stimulate weight loss. Whichever diet a client follows, is less important than their ability to maintain it. Also, their personal preferences and circumstances should be considered when suggesting a regimen. Behavioural modification and education by nurses are important factors in supporting these changes.
The call for nursing
Evidence supports the benefits of practice nurse-led clinics in both multifaceted and targeted approaches in CVD risk management and rehabilitation, with reductions in all-cause mortality, cardiac mortality, cardiac readmissions, non-fatal reinfarctions and improvements in BP, cholesterol, diet, weight and exercise.4, 5
This is especially true when interventions incorporate all four self-regulation techniques such as goal-setting, self-monitoring, planning and feedback.5 Other beneficial behaviour-change techniques include eating style (slowing down, and “mindful” eating), behaviour contracting (rewards for achieving a weight-loss goal), cognitive restructuring (using positive self-talk such as choosing to exercise after eating a piece of cake, rather than self-blaming), and problem-solving (developing strategies for managing tempting situations, such as parties).105

Such multi-faceted interventions usually include education, exercise training and psychological support. These can also be effectively supported by telephone counselling interventions.4
The tool included with this article can be used in one-to-one client education, using the client’s cholesterol profile, to illustrate the effects of various foods on serum cholesterol, CVD and diabetes risk, and making personalised recommendations for dietary change.
Alternatively, in a group educational setting, the rows, which indicate how certain dietary patterns will affect serum lipids and CVD risk, may be most helpful. For others, especially those asking about currently “popular” diets, the information in the dietary patterns section would be more useful.
People are all different — try to work with them to find individualised healthy solutions.
The original version of the table has anecdotally proved suitable for targeted secondary prevention of CVD, in coronary care and cardiac rehabilitation settings, particularly with those clients that have some prior knowledge of lipid profile and those who express scepticism about “low cholesterol” diets.
Other possible settings include, but are not limited to, practice nursing and specialist diabetes education.
The key message is that whatever diet people choose to follow, it is important to prioritise healthy plant-based fats and proteins, fresh fruits and vegetables and unrefined/minimally processed carbohydrates. People are all different — try to work with them to find individualised healthy solutions.
If you choose to use the table in your practice, please email the lead author with comments and suggestions. Jo Janssen can be reached at: [email protected]
The two pages of the table can be downloaded and laminated together as a handy resource to use with clients or for staff education. It is advisable to also keep a copy of the full article on hand for more detailed information, to support the abridged points in the table. The tool is available in A4 format for laminating here.
Jo Janssen, RN, MN, has been working for Nelson Marlborough Institute of Technology as a nursing tutor for 32 years and for Wairau Hospital as a high dependency unit nurse for 30 years. She has been reviewing and interpreting research findings on the effects of diet on cardiovascular disease for 20 years, following on from her master of nursing studies.
Denise Taylor, PhD, MSc, FRPharmS, FFRPS, MCMHP, FHEA, Reg Pharm NZ, is a senior lecturer at Te Kura Tapuhi Hauora — the School of Nursing, Midwifery, and Health Practice, at Victoria University of Wellington.
* This article was reviewed by Leanne Barclay, clinical nurse specialist in cardiac rehabilitation, Dunedin Hospital.
References
- Catapano, A. L., Graham, I., De Backer, G., Wiklund, O., Chapman, M. J., Drexel, H., … Zamorano, J. L. (2016). 2016 ESC/EAS Guidelines for the management of dyslipidaemias. European Heart Journal, 37, 2999–3058.
- Hooper, L., Martin, N., Jimoh, O. F., Kirk, C., Foster, E., & Abdelhamid, A. S. (2020). Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews 8(8), CD011737.
- Janssen, J. (2006). Fat simple — A nursing tool for client education. Nursing Praxis in New Zealand, 22(2), 21-32.
- Moola, S. (2021). Cardiovascular Disease: Dietary Pulses. Joanna Briggs Institute.
- Mbinji, M. (2021). Cardiovascular disease (primary and secondary prevention): lifestyle interventions. Joanna Briggs Institute.
- Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., Himmelfarb, C. D., Khera, A., Lloyd-Jones, D., McEvoy, J. W., Michos, E. D., Miedema, M. D., Munoz, D., Smith, S. C., Virani, S. S., Williams, K. A., Yeboah, J., & Ziaeian, B. (2019). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 140(11), e596–e646.
- Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., … Yeboah, J. (2018). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology ,73(24), 3168-3209.
- Mozaffarian D. (2016). Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation, 133(2), 187-225.
- National Heart Foundation of New Zealand (NHFNZ). (2021) Nuts, seeds and heart health.
- Chowdhury, R., Warnakula, S., Kunutsor, S., Crowe, F., Ward, H. A., Johnson, L., … Di Angelantonio, E. (2014). Association of dietary, circulating, and supplementary fatty acids with coronary risk: A systematic review and meta-analysis. Annuals of Internal Medicine, 160(6), 398-406.
- Malik, V. S., Chiuve, S. E., Campos, H., Rimm, E. B., Mozaffarian, D., Hu, F. B., & Sun, Q. (2015). Circulating very-long-chain saturated fatty acids and incident coronary heart disease in US men and women. Circulation, 132(4), 260-268.
- Steur, M., Johnson, L., Sharp, S. J., Imamura, F., Sluijs, I., Key, T. J., , … Forouhi, N. G. (2021). Dietary fatty acids, macronutrient substitutions, food sources and incidence of coronary heart disease: Findings from the EPIC-CVD Case-Cohort Study Across Nine European Countries. Journal of the American Heart Association, 10(23), e019814.
- Wang, D. D., Li, Y., Chiuve, S. E., Stampfer, M. J., Manson, J. E., Rimm, E. B., … Hu, F. B. (2016). Association of specific dietary fats with total and cause-specific mortality. JAMA Internal Medicine, 176(8),1134-45.
- Ministry of Primary Industries (MPI). (2024). How do trans-fatty acids affect your health?
- Abdelhamid, A. S., Martin, N., Bridges, C., Brainard, J. S., Wang, X., Brown, T. J., Hanson, S., Jimoh, O. F., Ajabnoor, S. M., Deane, K., Song, F., & Hooper, L. (2018). Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, 7, CD012345.
- Abdelhamid, A. S., Brown, T. J., Brainard, J. S., Biswas, P., Thorpe, G. C., Moore, H. J., Deane, K., Summerbell, C., Worthington, H., Song, F., & Hooper, L. (2020). Omega‐3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Library.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. (2015). 2015–2020 Dietary Guidelines for Americans. 8th Edition.
- Schwingshackl, L., & Hoffmann, G. (2014). Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids in Health and Disease, 13, 154.
- Guasch-Ferré, M., Zong, G., Willett, W. C., Zock, P. L., Wanders, A. J., Hu, F. B., & Sunformat, Q. (2019). Associations of monounsaturated fatty acids from plant and animal sources with total and cause-specific mortality in two US prospective cohort studies. AHA/ASA Journals, 124(8).
- Cao, X., Xia, J., Zhou, Y., Wang, Y., Xia, H., Wang, S., … Sun, G. (2022). The effect of MUFA-rich food on lipid profile: A meta-analysis of randomized and controlled-feeding trials. Foods, 11(13), 1982.
- Guasch-Ferré, M., Babio, N., Martínez-González, M. A., Corella, D., Ros, E., Martín-Peláez, S., … Salas-Salvadó, J., PREDIMED Study Investigators. (2015). Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Amerian Journal of Clinical Nutrition, 102(6),1563-73.
- Oghbaei, M., Prakash, J., & Yildiz, F. (2016). Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food & Agriculture, 2(1).
- Prasadi, N.V.P., & Joye, I. J. (2020). Dietary fibre from whole grains and their benefits on metabolic health. Nutrients, 12(10), 3045.
- Bergwall, S., Johansson, A., Sonestedt, E., & Acosta, S. (2022). High versus low –added sugar consumption for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, 1(1).
- Li, Y., Hruby, A., Bernstein, A. M., Ley, S. H., Wang, D. D., Chiuve, S. E., … Hu, F. B. (2015). Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. Journal of the American College of Cardiology, 66(14), 1538-1548.
- Jovanovski, E., Yashpal, S., Komishon, A., Zurbau, A., Blanco Mejia, S., Ho, H. V. T., … Vuksan V. (2018). Effect of psyllium (Plantago ovata) fiber on LDL cholesterol and alternative lipid targets, non-HDL cholesterol and apolipoprotein B: A systematic review and meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition, 108(5), 922-932.
- Reynolds, A., Mann, J., Cummings, J., Winter, N., Mete, E., & Te Morenga, L. (2019). Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet, 393(10170), 434-445.
- Hartley, L., May, M. D., Loveman, E., Colquitt, J. L., & Rees, K. (2016). Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, 2016(1), CD011472.
- Xi, B., Veeranki, S. P., Zhao, M., Ma, C., Yan, Y., & Mi, J. (2017). Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. Journal of the American College of Cardiology, 70(8), 913-922. https://doi.org/10.1016/j.jacc.2017.06.054
- Vu, K. N., Ballantyne, C. N., Hoogeveen, R. C., Nambi, V., Volcik, K. A., Boerwinkle, E., & Morrison, A.C. (2016). Causal role of alcohol consumption in an improved lipid profile: The Atherosclerosis Risk in Communities (ARIC) Study. PLOS ONE 11(2), e0148765.
- Mostofsky, E., Chahal, H. S., Mukamal, K. J., Rimm, E. B., Mittleman, M. A. (2015). Alcohol and immediate risk of cardiovascular events: A systematic review and dose-response meta-analysis. Circulation, 133(10), 979-87.
- Chikritzhs, T., Stockwell, T., Naimi, T., Andreasson, S., Dangardt, F., & Liang, W. (2015). Has the leaning tower of presumed health benefits from ‘moderate’ alcohol use finally collapsed? Addiction, 110(5), 726-7.
- Roerecke M. (2016). On bias in alcohol epidemiology and the search for the perfect study. Addiction, 112(2), 217-218.
- Wood, A. M., Kaptoge, S., Butterworth, A. S., Willeit, P., Warnakula, S., Bolton, T., … Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group. (2018). Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599912 current drinkers in 83 prospective studies. Lancet, 391(10129), 1513-1523.
- Oppenheimer, G. M., & Bayer, R. (2020). Is moderate drinking protective against heart disease? The science, politics and history of a public health conundrum. Milbank Quarterly, 98(1), 39-56.
- Catapano, A. L., Graham, I., De Backer, G., Wiklund, O., Chapman, M. J., Drexel, H., … Zamorano, J. L. (2016). 2016 ESC/EAS Guidelines for the management of dyslipidaemias. European Heart Journal, 37, 2999–3058.
- Mukamal, K. J. (2020). A safe level of alcohol consumption: The right answer demands the right question. Journal of Internal Medicine, 288(5), 550-559.
- World Health Organization (2021). Status report on alcohol consumption, harm and policy responses in 30 European countries 2019.
- Iakunchykova, O., Averina, M., Kudryavtsev, A. V., Wilsgaard, T., Soloviev, A., Schirmer, H., … Leon, D. A. (2020). Evidence for a direct harmful effect of alcohol on myocardial health: A large cross‐sectional study of consumption patterns and cardiovascular disease risk biomarkers from Northwest Russia, 2015 to 2017. Journal of the American Heart Association, 9(1), e014491.
- Raj, P., Zieroth, S., & Netticadan, T. (2015). An overview of the efficacy of resveratrol in the management of ischemic heart disease. Annals of the New York Academy of Science, 1348(1), 55-67.
- Weiskirchen, S., & Weiskirchen, R. (2016). Resveratrol: How much wine do you have to drink to stay healthy? Advances in Nutrition, 7, 706-18.
- Health Promotion Directorate, Te Whatu Ora – Health New Zealand. (2023). Standard drinks and legal limits.
- Lee, D. H., Rezende, L. F. M., Joh, H-K., Keum, N. N., Gerson Ferrari, F., Rey-Lopez, J. P., … Giovannucci, E. L. (2022). Long-Term Leisure-Time Physical Activity Intensity and All-Cause and Cause-Specific Mortality: A Prospective Cohort of US Adults. Circulation, 146(7), 523-534.
- Dombrowski, S. U., Knittle, K., Avenell, A., Araújo-Soares, V., & Sniehotta, F. F. (2014). Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ, 348, g2646.
- Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M-I., Corella, D., Fernando Arós, F., … Martínez-González, M. A. (2018). Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. New England Journal of Medicine, 378(25), e34.
- Guasch-Ferré, M., Liu, X., Malik, V. S., Sun, Q., Willett, W. C., Manson, J. E., … Bhupathiraju, S. N. (2017). Nut consumption and risk of cardiovascular disease. Journal of the American College of Cardiologists, 70(20), 2519-2532.
- Jayalath, V. H., de Souza, R. J., Sievenpiper, J. L., Ha, V., Chiavaroli, L., Mirrahimi, A., … Jenkins, D. J. (2014). Effect of dietary pulses on blood pressure: A systematic review and meta-analysis of controlled feeding trials. American Journal of Hypertension, 27(1), 56-64.
- National Heart Foundation of New Zealand. (2005). As cited in J. Janssen, (2006), Fat simple — A nursing tool for client education. Nursing Praxis in New Zealand, 22(2), 21-32.
- Willett, W. C., & Stampfer, M. J. (2003). Rebuilding the food pyramid. Scientific American, 288(1), 64-71.
- Mozaffarian, D., & Ludwig, D. S. (2015). The 2015 US Dietary Guidelines: Lifting the ban on total dietary fat. JAMA, 313(24), 2421-2422.
- Yang, Q., Lang, X., Li, W., & Liang., Y. (2022). The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: A systematic review and meta-analysis. European Journal of Clinical Nutrition, 76, 16-27.
- Augustin, L. S. A., Kendall, C. W. C., Jenkins, D. J. A., Willett, W. C., Astrup, A., Barclay, A. W., Bjorck, I., Brand-Miller, J. C., Brighenti, F., Buyken, A. E., Ceriello, A., La Vecchia, C., Livesey, G., Liu, S., Riccardi, G., Rizkalla, S. W., Sievenpiper, J. L., Trichopoulou, A., Wolever, T. M. S., Baer-Sinnott, S., & Poli, A. (2015). Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the International Carbohydrate Quality Consortium (ICQC). Nutrition, Metabolism and Cardiovascular Diseases: NMCD, 25(9), 795-815.
- Jenkins, D. J. A., Dehghan, M., Mente, A., Bangdiwala, S. I., Rangarajan, S., Srichaikul, K., … Yusuf, S., & Epub PURE Study Investigators. (2021). Glycemic index, glycemic load, and cardiovascular disease and mortality. New England Journal of Medicine, 384(14), 1312-1322.
- Livesey, G. & Livesey, H. (2019). Coronary heart disease and dietary carbohydrate, glycemic index, and glycemic load: Dose-response meta-analyses of prospective cohort studies. Mayo Clinic Proceedings — Innovations, Quality & Outcomes, 3(1), 52-69.
- Powell-Wiley, T. M., Poirier, P., Burke, L. E., Després, J. P., Gordon-Larsen, P., Lavie, C. J., … St-Onge, M. P., & American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. (2021). Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation, 143(21), e984-e1010.
- Barclay, A. W., Augustin, L. S. A., Brighenti, F., Delport, E., Henry, C. J., Sievenpiper, J. L., Usic, K., Yuexin, Y., Zurbau, A., Wolever, T. M. S., Astrup, A., Bullo, M., Buyken, A., Ceriello, A., Ellis, P. R., Vanginkell, M.-A., Kendall, C. W. C., La Vecchia, C., … Brand-Miller, J. (2021) Dietary glycaemic index labelling: A global perspective. Nutrients, 13(9), 3244.
- Bhupathiraju, S. N., Tobias, D. K., Malik, V. S., Pan, A., Hruby, A., Manson, J., Willett, W. C., & Hu, F. B. (2014). Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis 1–3. American Journal of Clinical Nutrition, 100(1), 218-232.
- Rossi, M., Turati, F., Lagiou, P., Trichopoulos, D., Augustin, L. S., La Vecchia, C., & Trichopoulou, A. (2013). Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: Results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC). Diabetologia, 56(11), 2405-2413.
- Atkins. (2021). Keto diet — the Atkins Way.
- Joseph, J. J., Deedwania, P., Acharya, T., Aguilar, D., Bhatt, D. L. Chyun, D. A., … Sperling, L. S. on behalf of the American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; and Council on Hypertension. (2022). Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: A Scientific Statement From the American Heart Association. Circulation, 145(9), e722-e759.
- Taubes, G. (2021). The case for keto. Rethinking weight control and the science and practice of low-carb/high fat eating. Penguin Random House.
- Darand, M., Hassanizadeh, S., Talebi, S., Darabi, Z., Bagherniya, M., Yaghoubi, F., … Abdollahzad, H. (2023). Comparison of the effect of a low-carbohydrate diet with a low-fat diet on anthropometric indices and body fat percentage: A systematic review and meta-analysis of randomized controlled trials. Journal of Nutrition and Food Security, 8(3), 493-520.
- Naude, C. E., Brand, A., Schoonees, A., Nguyen, K. A., Chaplin, M., & Volmink, J. (2022). Low-carbohydrate versus balanced-carbohydrate diets for reducing weight and cardiovascular risk. Cochrane Database of Systematic Reviews, 1(1), CD013334.
- Ghorbani, Z., Kazemi, A., Shoaibinobarian, N., Taylor, K., & Noormohammadi, M. (2023). Overall, plant-based, or animal-based low carbohydrate diets and all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Ageing Research Reviews, 90.
- Drummen, M., Tischmann, L., Gatta-Cherifi, B., Adam, T., & Westerterp-Plantenga, M. (2018). Dietary protein and energy balance in relation to obesity and co-morbidities. Frontiers in Endocrinology, 9, 443.
- Drummen, M., Tischmann, L., Gatta-Cherifi, B., Fogelholm, M., Raben, A., Adam, T. C., & Westerterp-Plantenga, M. S. (2020). High compared with moderate protein intake reduces adaptive thermogenesis and induces a negative energy balance during long-term weight-loss maintenance in participants with prediabetes in the postobese state: A PREVIEW study. Journal of Nutrition, 150(3), 458-463.
- Röhling, M., Kempf, K., Banzer, W., Braumann, K. M., Führer-Sakel, D., Halle, M., … & Predel, H.-G. (2022). A high-protein and low-glycemic formula diet improves blood pressure and other hemodynamic parameters in high-risk individuals. Nutrients, 14(7), 1443.
- Tettamanzi, F., Bagnardi, V., Louca, P., Nogal, A., Monti, G. S., Mambrini, S. P., … Menni, C. (2021). A high protein diet is more effective in improving insulin resistance and glycemic variability compared to a Mediterranean diet — A cross-over controlled inpatient dietary study. Nutrients, 13(12), 4380.
- Yu, Z., Nan, F., Wang, L. Y., Jiang, H., Chen, W., Jiang, Y. (2020). Effects of high-protein diet on glycaemic control, insulin resistance, and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Clinical Nutrition, 39(6), 1724-1734.
- de Carvalho, K. M., Pizato, N., Botelho, P. B., Dutra, E. S., & Gonçalves, V. S. (2020). Dietary protein and appetite sensations in individuals with overweight and obesity: A systematic review. European Journal of Nutrition, 59, 2317-2332.
- Oliveira, C. L. P., Boulé, N. G., Sharma, A. M., Elliott, S. A., Siervo, M., Ghosh, S., … Prado, C. M. (2021). A high-protein total diet replacement increases energy expenditure and leads to negative fat balance in healthy, normal-weight adults. American Journal of Clinical Nutrition, 113(2), 476-487.
- Nunes, C. L., Casanova, N., Francisco, R., Bosy-Westphal, A., Hopkins, M., Sardinha, L. B., & Silva, A. M. (2022). Does adaptive thermogenesis occur after weight loss in adults? A systematic review. British Journal of Nutrition, 127(3), 451-469.
- Spreckley, M., Seidell, J., & Halberstadt, J. (2021).Perspectives into the experience of successful, substantial long-term weight-loss maintenance: a systematic review. International Journal of Qualitative Studies on Health and Well-being, 16(1), 1862481.
- Hsu, K. J., Chien, K. Y., Tsai, S. C., Tsai, Y. S., Liao, Y. H., Chen, J. J., … Chen, C. N. (2021). Effects of exercise alone or in combination with high-protein diet on muscle function, aerobic capacity, and physical function in middle-aged obese adults: A randomized controlled trial. Journal of Nutrition, Health & Aging, 25(6), 727-734.
- Song, M., Fung, T. T., Hu, F. B., Willett, W. C., Longo, V. D., Chan, A. T., & Giovannucci, E. L. (2016). Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Internal Medicine, 176(10), 1453-1463. (Erratum in: JAMA Internal Medicine, 2016, Nov 1, 176(11), 1728.)
- Dinu, M., Pagliai, G., Casini, A., & Sofi, F. (2018). Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. European Journal of Clinical Nutrition, 72(1), 30-43.
- Laffond, A., Rivera-Picón, C., Rodríguez-Muñoz, P. M., Juárez-Vela, R., de Viñaspre-Hernández, R. R., Navas-Echazarreta, N., & Sánchez-González, J. L. (2023). Mediterranean diet for primary and secondary prevention of cardiovascular disease and mortality: An updated systematic review. Nutrients, 15(15), 3356.
- Rees, K., Takeda, A., Martin, N., Ellis, L., Wijesekara, D., Vepa, A., Das, A., Hartley, L., & Stranges, S. (2019). Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, 3(3), CD009825.
- Ahmad, S., Moorthey, M. V., Demler, O. V., Hu, F. B., Ridker, P. M., Chasman, D. I., & Mora, S. (2018). Assessment of risk factors and biomarkers associated with risk of cardiovascular disease among women consuming a Mediterranean diet. JAMA Network Open, 1(8), e185708.
- Ocagli, H., Berti, G., Rango, D., Norbiato, F., Chiaruttini, M. V., Lorenzoni, G., & Gregori, D. (2023). Association of vegetarian and vegan diets with cardiovascular health: An umbrella review of meta-analysis of observational studies and randomized trials. Nutrients, 15(19), 4103.
- Funmilola, B., Ayobami, A., Favour, D., Kolajo, B. A., Alexsandra, U., Omotola, A., … Anugwom Gibson, O. (2022). A comprehensive review on the effects of vegetarian diets on coronary heart disease. Cureus, 14(10), e29843.
- United States Department of Agriculture. (2022). Scientist evaluates dietary food patterns for healthy adults eating dairy-free vegetarian or vegan diets.
- Satija, A., Bhupathiraju, S. N., Spiegelman, D., Chiuve, S. E., Manson, J. E., Willett, W., … Hu, F. B. (2017). Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. Journal of the American College of Cardiology, 70(4), 411-422.
- Rees, K., Al-Khudairy, L., Takeda, A., & Stranges, S. (2021). Vegan dietary pattern for the primary and secondary prevention of cardiovascular diseases. Cochrane Database of Systematic Reviews, 2(2), CD013501.
- Satija, A., & Hu, F. B. (2018). Plant-based diets and cardiovascular health. Trends in Cardiovascular Medicine, 28(7), 437-441.
- Anic, K., Schmidt, M. W., Furtado, L., Weidenbach, L., Battista, M. J., Schmidt, M., Schwab, R., Brenner, W., Ruckes, C., Lotz, J., Lackner, K. J., & Hasenburg, A. (2022). Intermittent fasting — short- and long-term quality of life, fatigue, and safety in healthy volunteers: A prospective, clinical trial. Nutrients, 14, 4216.
- Aragon, A. A., & Schoenfeld, B. J. (2022). Does timing matter? A narrative review of intermittent fasting variants and their effects on bodyweight and body composition. Nutrients, 14(23), 5022.
- Zhang, Q., Zhang, C., Wang, H., Ma, Z., Liu, D., Guan, X., Liu, Y., Fu, Y., Cui, M., & Dong, J. (2022). Intermittent fasting versus continuous calorie restriction: Which is better for weight loss? Nutrients, 14(9), 1781.
- Liu, L., Chen, W., Wu, D., & Hu, F. (2022). Metabolic efficacy of time-restricted eating in adults: A systematic review and meta-analysis of randomized controlled trials. The Journal of Clinical Endocrinology & Metabolism, 107(12), 3428–3441.
- Santos, H. O., & Macedo, R. C. O. (2018). Impact of intermittent fasting on the lipid profile: Assessment associated with diet and weight loss. Clinical Nutrition ESPEN, 24, 14-21.
- Longo, V. D., & Panda, S. (2016). Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabolism, 23(6), 1048-1059.
- Varady, K. A., Cienfuegos, S., Ezpeleta, M., & Gabel, K. (2021). Cardiometabolic benefits of intermittent fasting. Annual Review of Nutrition, 41, 333–361.
- Mosley, M., & Spencer, M. (2023). The 5:2 fast diet.
- Torres, L., Lee, J. L., Park, S., Di Lorenzo, R. C., Branam, J. P., Fraser, S. A., & Salisbury, B. A. (2022). Retention, fasting patterns, and weight loss with an intermittent fasting app: Large-scale, 52-week observational study. JMIR Mhealth Uhealth, 10(10), e35896
- Snijders, T., Trommelen, J., Kouw, I. W. K., Holwerda, A. M., Verdijk, L. B., & van Loon, L. J. C. (2019). The impact of pre-sleep protein ingestion on the skeletal muscle adaptive response to exercise in humans: An update. Frontiers in Nutrition, 6, 17.
- Williamson, E., & Moore, D. R. (2021). A muscle-centric perspective on intermittent fasting: A suboptimal dietary strategy for supporting muscle protein remodeling and muscle mass? Frontiers in Nutrition, 8, 640621.
- Moro, T., Tinsley, G., Bianco, A., Marcolin, G., Pacelli, Q. F., Battaglia, G., Palma, A., Gentil, P., Neri, M., & Paoli, A. (2016). Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. Journal of Translational Medicine, 14(1), 290.
- Tinsley, G. M., Moore, M. L., Graybeal, A. J., Paoli, A., Kim, Y., Gonzales, J. U., … & Cruz, M. R. (2019). Time-restricted feeding plus resistance training in active females: a randomized trial. American Journal of Clinical Nutrition, 110(3), 628-640.
- Moore, D. R., Churchward-Venne, T. A., Witard, O., Breen, L., Burd, N. A., Tipton, K. D., & Phillips, S. M. (2015). Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. Journals of Gerontology, Series A, 70(1), 57-62.
- Tobias, D. K., Chen, M., Manson, J.E., Ludwig, D. S., Willett, W., & Hu, F. B. (2015). Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes and Endocrinology, 3(12), 968-79.
- Buso, M. E. C., Seimon, R. V., McClintock, S., Muirhead, R., Atkinson, F. S., Brodie, S., … Sainsbury, A. (2021). Can a higher protein/low glycemic index vs. a conventional diet attenuate changes in appetite and gut hormones following weight loss? A 3-year PREVIEW sub-study. Frontiers in Nutrition, 8, 640538.
- Willett, W.C. (2018). Diet and health — finding a path to Veritas. European Journal of Epidemiology, 33, 127-135.
- Tangney, C.C. & Rosenson, R.S. (2024). Lipid management with diet or dietary supplements. UpToDate.
- National Heart Foundation of New Zealand (NHFNZ). (2024). 7 Foods that may lower your cholesterol.
- Raynor, H. A., & Champagne, C. M. (2016). Position of the Academy of Nutrition and Dietetics: Interventions for the Treatment of Overweight and Obesity in Adults. Journal of the Academy of Nutrition and Dietetics, 116(1), 129-147.




