Focus on medicines equity
This article is the second in a series supplied by He Ako Hiringa to support nurses’ professional development.
He Ako Hiringa is a clinical education programme for primary care clinicians, funded by PHARMAC Te Pātaka Whaioranga to raise awareness of health inequities and improve access to funded medicines. Priority populations include Māori, Pacific peoples, rural communities, former refugees, and those living in high socioeconomic deprivation.
He Ako Hiringa is focusing on four conditions significantly amenable to treatment with medicine: asthma, cardiovascular disease, diabetes and gout. Five main drivers of medicine access equity have been identified: medicine availability, accessibility, affordability, acceptability and appropriateness. Nurses can have a huge impact on these drivers.

These new agents can reduce mortality and cardiovascular events, including heart failure, and reduce progression of renal failure. As of February 1, 2021, the SGLT2 inhibitor empagliflozin is funded for type 2 diabetes in New Zealand under Special Authority. The GLP-1 receptor agonist dulaglutide will also be funded, once approved by Medsafe.
Since nurses play a pivotal role in supporting patients with diabetes, they will need to become familiar with these medications and their place in management of type 2 diabetes.
Background
New Zealand population
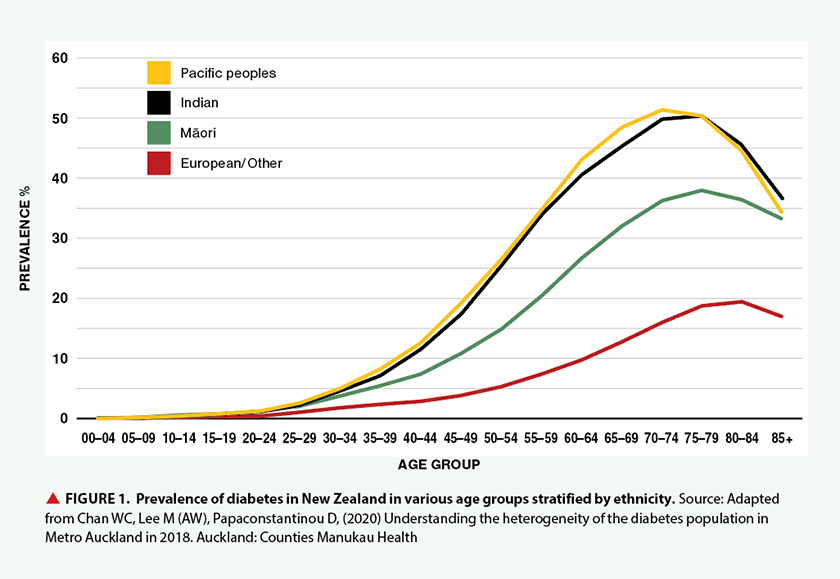
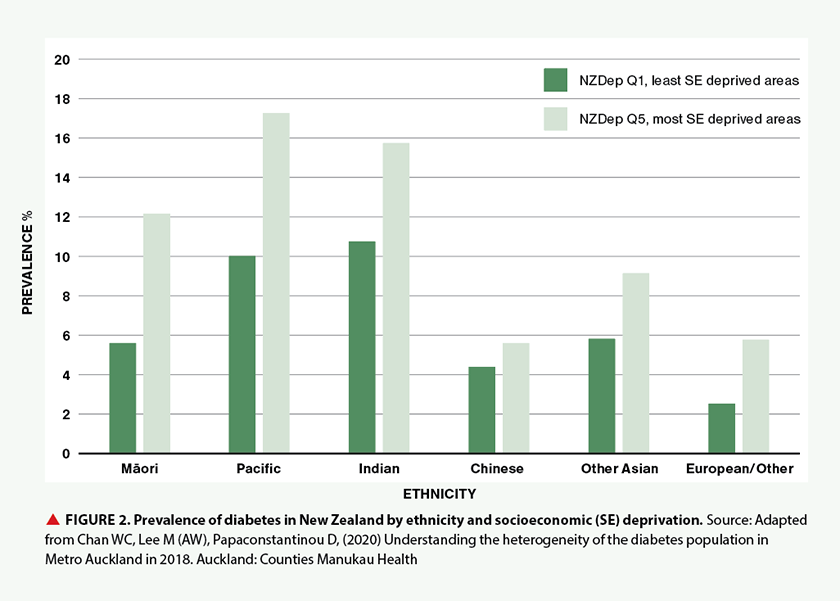
More than 250,000 people have diabetes in New Zealand, with 90 per cent of these estimated to have type 2 diabetes. The prevalence of type 2 diabetes increases with age and parallels the increased population prevalence of obesity, with known risk factors such as a calorific diet, low physical activity, family history and genetics. People of Pacific, Indian and Māori ethnicities are affected at an earlier age than those of European ethnicity. At the age of 55, the proportion of people with diabetes is approximately 35 per cent in people of Pacific and Indian ethnicity, 20 per cent in Māori and eight per cent in New Zealand Europeans (see Figure 1). Although socioeconomic deprivation is associated with an increase in diabetes prevalence, there still appears to be a higher prevalence among people of non-European ethnicity, particularly Pacific peoples, Māori and people of Indian ethnicity (see Figure 2).
Sustained hyperglycaemia leads to microvascular and macrovascular complications, which are amplified by other cardiovascular risk factors, such as elevated blood pressure and lipid levels, smoking, renal disease and increased age. Almost universal screening for diabetes as part of annual cardiovascular risk assessments means that more new cases of type 2 diabetes are screen-detected, rather than symptomatically diagnosed many years after diabetes onset. Unfortunately, the proportion of people with poor diabetes control still appears to be rising and an increasing number of people with diabetes require dialysis, and experience cardiovascular events and premature death. Māori and Pacific peoples are over-represented in the proportion of patients with type 2 diabetes and poor glucose control, and in those who experience complications of diabetes.
Classification and diagnosis of diabetes
Diabetes is indicated by an elevated HbA1c (glycated haemoglobin) test or elevated fasting glucose, with or without symptoms of diabetes such as polyuria and polydipsia, blurred vision, weight loss and fatigue. In asymptomatic cases, two HbA1c readings above 50mmol/mol (or fasting glucose above 7mmol/L) are required to diagnose diabetes. When symptoms are present, one laboratory test is diagnostic. Careful enquiry will often reveal a history of osmotic symptoms. After diagnosis of diabetes, correct classification is critical for ensuring people receive appropriate treatment and management.
Type 1 diabetes is caused by the autoimmune destruction of pancreatic beta cells, and results in severe insulin deficiency. Insulin replacement (usually with multiple daily injections or through an insulin pump) is required. The presentation of type 1 diabetes may be latent – an absence of a clear family history of type 2 diabetes and poor response to oral therapy may indicate latent autoimmune diabetes in adults (LADA), especially in the slimmer patient. The presence of glutamic acid decarboxylase antibodies will confirm diagnosis and the patient should be referred for specialist investigation.
Patients with type 2 diabetes continue to produce substantial amounts of their own insulin and therefore respond to non-insulin therapy and have more stable glycaemia. In type 2 diabetes, hyperglycaemia occurs due to a combination of metabolic factors that culminate in insulin resistance and eventual insulin deficiency, as the pancreatic beta cells no longer produce sufficient insulin to compensate. Excessive insulin production in response to obesity-related insulin resistance can result in visceral deposition of fat, contributing to fatty liver disease. People with type 2 diabetes often have a reduced incretin response to food. Incretin hormones, such as GLP-1, are usually secreted by the intestinal mucosa and enhance insulin secretion, slow gastric emptying, increase satiety, and inhibit the action of glucagon (glucagon stimulates glycogenolysis and increases blood glucose levels).2
Type 2 diabetes usually requires progressively more treatments
As insulin production declines, progressively more treatments are required. Weight loss through lifestyle changes, as well as glucose-lowering therapies and other medications targeting cardiovascular risk, can lower morbidity and mortality in people with type 2 diabetes.
Recent cardiovascular and renal outcome trials show SGLT2 inhibitor and GLP-1 receptor agonist medications provide multifactorial benefits in patients with diabetes. These agents can reduce cardiovascular disease (CVD), renal disease, heart failure and mortality when used alongside existing therapies, and international guidelines1 now recommend these disease-modifying treatments be prescribed for appropriate patients.
It is important to consider these new medications for all people with type 2 diabetes, alongside existing medications, lifestyle modification, low-energy diets and bariatric surgery.
General practice teams need to become confident in introducing these medications to patients with CVD, renal disease or risk factors for these.
It is critical that health-care providers consider SGLT2 inhibitors as organ-protective agents rather than merely glucose-lowering drugs.
New diabetes drugs have significant cardiovascular and renal benefits

Metformin and lifestyle change remain the standard management approach in patients with a new-onset diabetes diagnosis. However, updated management guidelines recommend the use of SGLT2 inhibitor and GLP-1 receptor agonist medications to produce significant cardiovascular and renal benefits, in addition to their glucose-lowering ability. These beneficial effects appear to be independent of the magnitude of glucose lowering and are additional to other desirable effects of these medications in promoting weight loss and a low risk of hypoglycaemia (see Panel 1).
The main change in the international guidelines1 is the removal of a need for glycaemic control to add a medication with cardio-renal benefit; however, there is an HbA1c requirement for funded use of these medications in New Zealand for rationing purposes (see Panel 2).
The beneficial effects of these drugs do not appear to be mediated through glucose lowering and are seen irrespective of patients’ HbA1c level at baseline. In fact, there is emerging evidence that benefits in chronic kidney disease (CKD) and CVD occur even in patients without diabetes.
The main advantage of these drugs and reasons for using them is their beneficial effects on heart failure, kidney disease and cardiovascular death, with the positive side benefit on blood glucose.
SGLT2 inhibitors protect organs
SGLT2 inhibitors decrease renal absorption of glucose, leading to increased excretion of glucose, weight loss of 2-4kg and modest lowering of blood pressure. There is no hypoglycaemic risk unless they are used with other medications that could cause hypoglycaemia. SGLT2 inhibitors appear to have a class effect on reducing glucose, heart failure and renal function decline, and empagliflozin has demonstrated cardiovascular benefits. This class of medications also lowers serum uric acid and reduces the incidence of gout by 40 per cent in observational studies.
It is not yet fully understood how these medications produce the unexpected benefits for the heart and kidneys, although it is thought to be related to natriuresis (sodium excretion via the kidneys). It is critical that health-care providers consider SGLT2 inhibitors as organ-protective agents rather than merely glucose-lowering drugs.
Starting SGLT2 inhibitors
Care is needed to avoid volume depletion, and there is an increased risk of genital fungal infections (approximately four per cent incidence) and a rare risk of ketoacidosis with normal blood glucose concentrations (approximately 0.1 per cent incidence). These medications should therefore be stopped if the patient is fasting or severely unwell. Patients need to be reminded to seek medical attention and must have their ketones checked if they present feeling unwell with nausea, vomiting or abdominal pain.
Panel 1. SGLT2 inhibitor or GLP-1 receptor agonist:
Which to choose:
- There is a clear benefit for SGLT2 inhibitors in reduction of heart failure compared with GLP-1 receptor agonists, which produce no direct benefits on heart failure.
- Clear renal benefits are seen with SGLT2 inhibitors, and results from several GLP-1 receptor agonists studies have shown reduced macroalbuminuria and slower eGFR decline.
- Patients with moderate to severe CKD will benefit from glucose-lowering and slowing of eGFR decline with GLP-1 receptor agonists.
- In moderate to severe CKD, SGLT2 inhibitors will slow renal decline but not lower glucose.
- Patients wishing to lose weight would benefit from GLP-1 receptor agonists over SGLT2 inhibitors.
- Dulaglutide is available as a once-weekly preparation. Teaching injection technique should not be a deterrent, as this is very simple with automated pens, tiny needles and simple dosing.
Dose adjustment of background diabetes medications
When adding an SGLT2 inhibitor, there is no need to adjust the dose of background treatment with metformin or dipeptidyl peptidase-4 (DPP-4) inhibitors, as the SGLT2 inhibitor will not cause hypoglycaemia itself. For patients already taking a sulfonylurea, the sulfonylurea dose may need to be reduced or stopped (if they develop hypoglycaemia). A patient already on insulin may require a 10-20 per cent reduction in insulin dose, depending on HbA1c and fasting glucose levels.
Use in renal disease
Cardiovascular benefits, including those related to heart failure, extend to patients with type 2 diabetes and estimated glomerular filtration rate (eGFR) reduced to 30mL/min/1.73m2. However, the glucose-lowering effects are seen largely in those with higher eGFR.3, 4, 5
GLP-1 receptor agonists: Don’t be deterred by injectables
GLP-1 is an incretin hormone synthesised and secreted by the gut in response to nutrient intake. It stimulates insulin secretion by pancreatic beta cells in response to rising blood glucose levels, promotes satiety and results in body weight loss, as well as a lowering of blood pressure and lipid levels.
How GLP-1 receptor agonists reduce cardiovascular events is unclear, as benefits are seen even in patients who don’t have changes in body weight or lowering of blood pressure or lipid levels. They likely act to decrease inflammation, to stabilise atherosclerotic plaque and prevent development and progression of atherosclerotic disease, as well as preserving renal function. Some, including dulaglutide and liraglutide, have proven cardiovascular benefits.
The main side effects are transient nausea, vomiting and diarrhoea, occurring in 10-20 per cent of patients, which usually subsides within one to two months. Injection-site reactions have been described. Experience is limited in the use of GLP-1 receptor agonists in patients with gastroparesis, so these drugs are not recommended for these patients.
Dose adjustment of background diabetes medications
When adding a GLP-1 receptor agonist, there is no need to adjust the dose of background treatment with metformin, as the GLP-1 receptor agonist will not cause hypoglycaemia itself. For patients taking a DPP-4 inhibitor, eg vildagliptin, the DPP-4 inhibitor should be stopped and replaced by the GLP-1 receptor agonist. If a patient is already on a sulfonylurea and a GLP-1 receptor agonist is added, the sulfonylurea dose may need reducing or stopping to prevent hypoglycaemia. A patient already on insulin may require a significant reduction in insulin dose when a GLP-1 receptor agonist is added, depending on HbA1c and fasting glucose levels.
Panel 2. Special Authority criteria for subsidy of empagliflozin, empagliflozin with metformin, or dulaglutide
Initial application is from any relevant practitioner (including GPs, nurse practitioners and pharmacist prescribers). Approvals valid without further renewal, unless notified, for applications meeting the following criteria:
All of the following:
- Patient has type 2 diabetes, and
- Any of the following:
- Patient is Māori or any Pacific ethnicity, or
- Patient has pre-existing cardiovascular disease or risk equivalent*, or
- Patient has an absolute five-year cardiovascular disease risk of 15% or greater according to a validated cardiovascular risk assessment calculator, or
- Patient has a high lifetime cardiovascular disease risk due to being diagnosed with type 2 diabetes during childhood or as a young adult, or
- Patient has diabetic kidney disease**, and
- Target HbA1c (of 53mmol/mol or less) has not been achieved despite the regular use of at least one blood-glucose lowering agent (eg metformin, vildagliptin or insulin) for at least three months, and
- SGLT2 therapy will not be funded if patient is taking a funded GLP-1, and vice versa.
Criteria 2.1-2.5 describe patients at high risk of cardiovascular or renal complications of diabetes.
* Defined as: prior cardiovascular disease event (ie angina, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, transient ischaemic attack, ischaemic stroke, peripheral vascular disease), congestive heart failure or familial hypercholesterolaemia.
** Defined as: persistent albuminuria (albumin:creatinine ratio greater than or equal to 3mg/mmol, in at least two out of three samples over a 3-6 month period) and/or eGFR less than 60ml/min/1.73m2 in the presence of diabetes, without alternative cause.
Summarised from PHARMAC press release, December 21, 20208
Use in renal disease
Dulaglutide can be used without dose adjustments in patients with mild or moderate renal impairment (eGFR >30mL/min). If a patient has type 2 diabetes and either CVD risk or CKD risk, including persistent microalbuminuria, they may benefit from an SGLT2 inhibitor or a GLP-1 receptor agonist.
What is the place of older diabetes drugs in the new NZSSD guidelines?
SGLT2 inhibitors and GLP-1 receptor agonists will add to the variety of diabetes medicines already in the health-care provider’s toolbox, some of which are described here.
Latest guidelines
Diabetes management guidelines, including insulin initiation, are available on Regional Health Pathways and an updated New Zealand Type 2 Diabetes Management Guidance is available on the New Zealand Society for the Study of Diabetes website.
DPP-4 inhibitors
DPP-4 inhibitors work by inhibiting the breakdown of endogenous incretin hormones. DPP-4 inhibitors increase GLP-1 twofold to threefold, whereas injectable GLP-1 receptor agonists increase GLP-1 eightfold to tenfold. DPP-4 inhibitors such as vildagliptin are increasingly replacing sulfonylureas as second-line agents, as they do not cause hypoglycaemia on their own and are weight neutral. However, DPP-4 inhibitors are generally less effective than sulfonylureas in lowering HbA1c.
While cardiovascular safety studies of vildagliptin have proven its safety, it is generally considered to have a neutral effect on CVD.6 Hence, in anyone with significant CVD, heart failure or renal risk, DPP-4 inhibitors should be considered a third-line treatment after SGLT2 inhibitors.
In patients without cardiovascular risk factors, heart failure or CKD, early combination therapy of vildagliptin with metformin should be considered. The VERIFY study7 indicated that patients who commenced dual therapy when HbA1c was 48-58mmol/mol had a longer period of time with a HbA1c <53 mmol/mol than those who received sequential therapy with vildagliptin after failing metformin monotherapy.
Sulfonylureas and thiazolidinediones
Sulfonylureas and thiazolidinediones (TZDs) are the last choice of drugs in the new European Union (EU) and United States (US) guidelines for type 2 diabetes.1 These therapies are used for glucose-lowering in situations where cost is a major issue and/or there is a reluctance to use insulin to control glycaemia. They have been widely used in New Zealand.
Sulfonylureas close potassium channels at the surface of the pancreatic beta cell and lead to insulin release. The major side effect of sulfonylureas is hypoglycaemia, as insulin is released irrespective of blood glucose levels. For this reason, patients must pay closer attention to managing the balance between carbohydrate intake and activity and will need to monitor their blood glucose levels.
Pioglitazone is the only drug still available in the TZD class. It binds to the nuclear peroxisome-proliferator-activated receptor and modulates gene expression, which improves insulin sensitivity. Pioglitazone does not cause hypoglycaemia and is preferred over sulfonylureas if there is a compelling need to minimise hypoglycaemia. Pioglitazone leads to triglycerides reduction and improved fatty liver disease and possibly lowers CVD events. However, pioglitazone therapy is frequently associated with weight gain (approximately 2–3kg) and is not suitable for patients with heart failure or macular oedema, or in patients who are at increased risk of fracture.
Insulin
Insulin should be considered for patients who do not achieve target HbA1c levels on other combination glucose-lowering therapies, but GLP-1 receptor agonists are preferred over insulin when possible. Due to the progressive failure of beta cells in type 2 diabetes, many patients will eventually need insulin therapy to achieve target glycaemic levels.
Earn 0.5 CPD hours
Nurses can claim 0.5 professional development hours for reading this article then answering the following questions:
- Describe the ethnic distribution of diabetes in New Zealand
- Explain the key differences between type 1 and type 2 diabetes
- Describe the benefits and adverse effects of SGLT2 inhibitors and GLP-1 receptor agonists
- Identify which groups of patients may benefit from treatment with the new medications, and understand the associated funding criteria
- Describe the place of older therapies in the treatment of type 2 diabetes

This article was originally written by Auckland District Health Board (DHB) endocrinologist and diabetologist Rinki Murphy and published in NZ Doctor late last year. It has subsequently been edited for Kai Tiaki Nursing New Zealand readership by Waitemata DHB diabetes clinical nurse specialist Lisa Sparks, and updated with the latest funding information.
Reference
- Buse, J., Wexler. D., Tsapas, A., Rossing, P., Mingrone, G., Mathieu, C., D’Alessio, D., & Davies, M. (2020). 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care, 43(2), 487–93.
- Hinnen D. (2017). Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectrum, 30(3), 202–210. doi:10.2337/ds16-0026
- Zinman, B., Wanner, C., Lachin, J., Fitchett, D., Bluhmki, E., Hantel, S., Mattheus, M., Devins, T., Johansen, O., Woerle, H., Broedl, U., & Inzucchi, S., for the EMPA-REG OUTCOME Investigators. (2015). Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine, 373, 2117–2128. doi:10.1056/NEJMoa1504720
- Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N., Shaw, W., Law, G., , Desai, M., & Matthews, D., for the CANVAS Program Collaborative Group. (2017). Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine, 377, 644–657. doi:10.1056/NEJMoa1611925
- Wiviott, S., Raz, I., Bonaca, M., Mosenzon, O., Kato, E., Cahn, A., Silverman, M., Zelniker, T., Kuder, J., Murphy, S., Bhatt, D., Leiter, L., McGuire, D., Wilding, J., Ruff, C., Gause-Nilsson, I., Fredriksson, M., Johansson, P., Langkilde, A.-M., & Sabatine, M., for the DECLARE–TIMI 58 Investigators. (2019). Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine, 380, 347–57. doi:10.1056/NEJMoa1812389
- Sinha, B., & Ghosal, S. (2019). Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Research and Clinical Practice, 150, 8-16. doi:10.1016/j.diabres.2019.02.014
- Matthews, D., Paldanius, P., Proot, P., Chiang, Y., Stumvoll, M., Del Prato, S., VERIFY study group. (2019). Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet, 394(10208), 1519-1529. doi:10.1016/S0140-6736(19)32131-2
- PHARMAC. (2020, Dec 21). PHARMAC to fund new diabetes medicines with amended Special Authority criteria (press release).