* Reading this article is the equivalent to one hour of CPD.
Overview
Most people associate type 2 diabetes with older adults. However increasing numbers of children and young people are developing it. High rates of child- and youth-onset type 2 diabetes in New Zealand present major challenges for affected individuals, their whānau and health professionals.
Young people with type 2 diabetes often struggle to manage glycaemic levels and are typically under-treated for hypertension, dyslipidaemia and smoking cessation. Youth with type 2 develop complications (particularly renal, retinopathy and cardiovascular disease) more quickly than adults with type 2 diabetes: half of them having significant complications within 10 to 15 years after diagnosis.
There is a lack of research for this young population and their management is often based on studies from adults with type 2 diabetes. Evidence on the effectiveness of lifestyle and pharmaceutical interventions and the rate of complications have largely come from two North American youth cohort studies: SEARCH and TODAY. (Research based on these two studies is referenced throughout this article.)
There is a lack of research for this young population and their management is often based on studies from adults with type 2 diabetes.
Both study groups have followed several hundred children and youth with type 2 diabetes from 2002 and 2004, respectively. Additionally, several randomised controlled trials have been undertaken to test interventions among these two study groups, to improve hyperglycaemia, hypertension and lipid levels, and to compare their effectiveness with adult trials in reducing diabetes-related complications.
This article describes:
- how obesity drives the development of type 2 diabetes in this age group,
- risk factors for screening,
- diabetes-related complications and comorbidities, and
- best management practices.
Challenges associated with managing glucose levels, and cardiovascular and renal risk factors in this age group are addressed, along with recommended lifestyle and pharmaceutical interventions.

Introduction
Globally, the incidence of type 2 diabetes has rapidly increased over the past 20 years. Among young people (under 20 years) in the United States (US), there are 13.8 new cases diagnosed each year for every 100,000 population.1 Youth from ethnic minority groups living in Western countries are two to eight times more likely to be diagnosed with the condition than their European counterparts.1
In Australia, the occurrence of diabetes is much higher in Indigenous youth (<17 years) (4.5 to 31 per 100,000/year) than other youth (<1.4 per 100,000/year).2 Similarly, in New Zealand, Pacific and Māori youth (<15 years) have a significantly higher annual rate of type 2 diabetes (5.9 and 4.1 per 100,000 children), respectively, than Asian and European youth (0.6 and 0.1 per 100,000).3
Girls have almost double the rate of boys and are most commonly diagnosed during mid-puberty, around the age of 13 years.3
Obesogenic environment: Growing levels of youth diabetes are occurring as modern urban life becomes increasingly obesogenic — that is an environment in which it is easier to gain weight and harder to lose it. The obesogenic environment is driven by an abundance of processed food, high in refined carbohydrates, salt and saturated fats and low in nutrients.4
In a largely unregulated profit-driven food industry, more unhealthy food outlets serve economically disadvantaged communities.5 In New Zealand, approximately 58 per cent of young people consume a third of their daily food intake at school.5 Only 17 per cent of New Zealand schools have a food-specific policy and most contract a for-profit food service with legislation loopholes allowing unhealthy food to be sold.5

A reluctance by successive governments to instigate policy changes to reduce the obesogenic environment exposes children and youth, mostly from economically disadvantaged backgrounds, to a diet that puts them at risk of obesity and developing type 2 diabetes.6
Risk factors for screening: Almost all youth with prediabetes and type 2 diabetes are significantly overweight or obese1 and are less physically active than others of the same age.7 In addition to the higher prevalence among Pacific and Māori youth, early determinants of health have the greatest impact on children and youth developing type 2 diabetes.
In New Zealand, 29 per cent of obese adolescents were from the most economically deprived households.8 This proportion is far higher for youth with type 2 diabetes, with 70 per cent in North America being from similarly economically deprived households.1
At least two-yearly screening is recommended for those at risk9 — this aligns with the American Diabetes Association (ADA) and UK-based National Institute for Health Care Excellence (NICE) guidelines.10
Diagnosis of youth-onset type 2 diabetes usually occurs during opportunistic routine primary care consultations, based on risk factors, signs and symptoms of diabetes, including acanthosis nigricans (dark velvety skin in body folds and creases),8 and is confirmed by an HbA1c >50 mmol/mol.9 It is recommended that Māori and Pacific children are screened, if obese, with one additional major risk factor.9
Insulin resistance (reduced insulin sensitivity), metabolic syndrome and prediabetes: Cells that require insulin for glucose entry are mostly energy storage cells (liver, adipose and skeletal muscle — outlined in Figure 1, below). In response to increased fatty tissue, these cells become insulin resistant, release inflammatory chemicals and are less able to take up glucose from the bloodstream.11
Liver cells respond to the reduction in intracellular glucose by breaking down stored glycogen into glucose that is secreted into the bloodstream, and altering lipid production. Synthesis of both the unhealthy lipids, low-density-lipoprotein-cholesterol (LDL-C) and very-low-density lipoprotein-cholesterol (VLDL-C), are increased, and healthy high-density-lipoprotein-cholesterol (HDL-C) synthesis is reduced.12
LDL-C is a major driver of atherosclerosis and VLDL-C delivers more fatty molecules to the insulin-producing beta cells in the pancreas, leading to insulin resistance in those cells and reduced secretion of insulin. Insulin resistant adipocytes (fat cells) release stored triglycerides (TAGs) into the bloodstream in response to reduced uptake of glucose.11
Inflammation, hypertension, and the adverse changes in lipid profiles referred to as dyslipidaemia, promote atherosclerosis and early-onset cardiovascular disease (Figure 1).
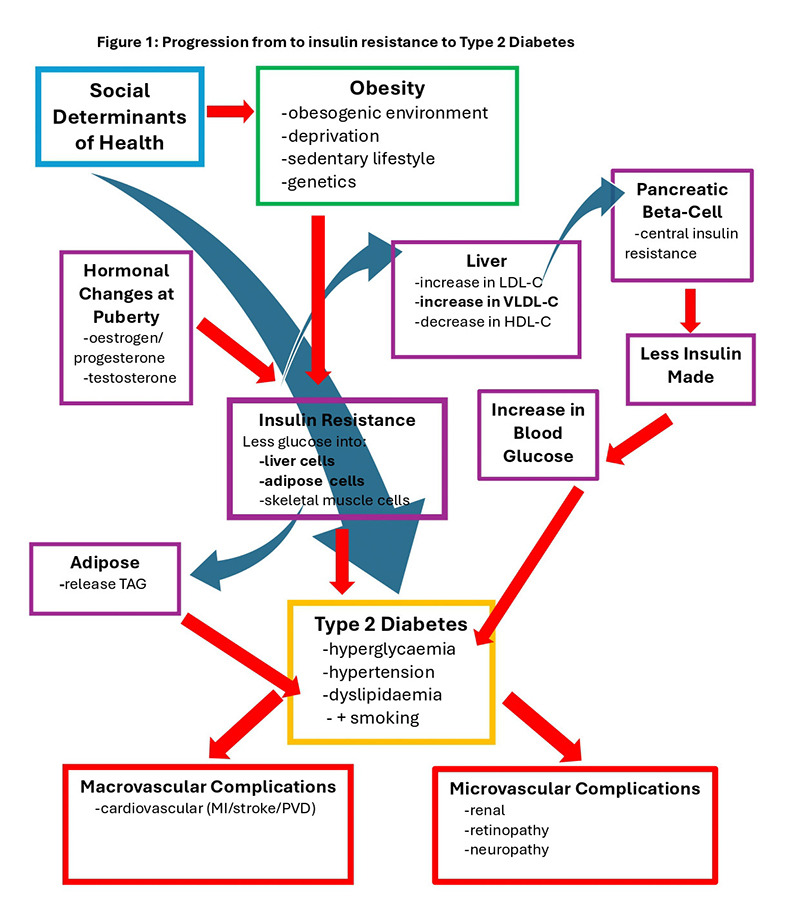
Insulin resistance and puberty: Insulin resistance, or reduced insulin sensitivity, is largely driven by excess adiposity (body fat) and is the main risk factor for developing type 2 diabetes in young people.
Increases in growth hormone, testosterone and oestrogen during puberty cause a slight increase in insulin resistance and elevated serum glucose levels, which, in adolescents who are already insulin-resistant, can lead to a diagnosis of type 2 diabetes.13
Girls are more likely to be diagnosed during puberty,13which coincides with a decrease in physical activity14 and wide hormonal variations associated with ovulation and menstruation.7
In New Zealand, less than a fifth of adolescents meet physical activity guidelines of at least one hour of moderate to vigorous activity daily,15 increasing the risk of youth-onset type 2 diabetes. Declining fitness from childhood to adolescence is also associated with developing type 2 diabetes as adults.16
Youth with type 2 develop complications (particularly renal, retinopathy and cardiovascular disease) more quickly than adults.
Associated health conditions: Acanthosis nigricans (hyperpigmented skin changes), non-alcoholic fatty liver disease,8 polycystic ovary syndrome (PCOS) and hyperandrogenism (elevated testosterone levels in females)17 are commonly seen in youth with metabolic syndrome or type 2 diabetes.
Depression, neurodevelopmental differences, eating disorders, poor quality sleep patterns and sleep apnoea are also more common in youth with type 2, reducing their quality of life.12 Although there are few reports on the prevalence of depression, including from New Zealand, the TODAY study reported almost 60 per cent of non-European youth had signs and symptoms of depression.18
Diabetes-related complications
Microvascular (capillary small vessel disease) and macro-vascular (atherosclerotic large vessel disease) lead to complications in youth with type 2, and at a significantly faster rate than adults with type 2 and youth with type 1, with 75 per cent developing at least one complication within eight years of diagnosis.7,19
Microvascular complications are common, with 55 per cent of the TODAY cohort developing renal disease, 51 per cent retinopathy and 32 per cent neuropathy within 14 years from diagnosis.20
Microvascular complications
Hyperglycaemia, hypertension and smoking are the major risk factors leading to diseased and dysfunctional capillaries and microvascular complications.11,21 Excessive glucose binds to cell membrane proteins on the endothelial cells lining the capillaries in the kidneys, retina and around peripheral nerve cells, forming advanced-glycation end-products causing structural changes, loss of integrity and function.
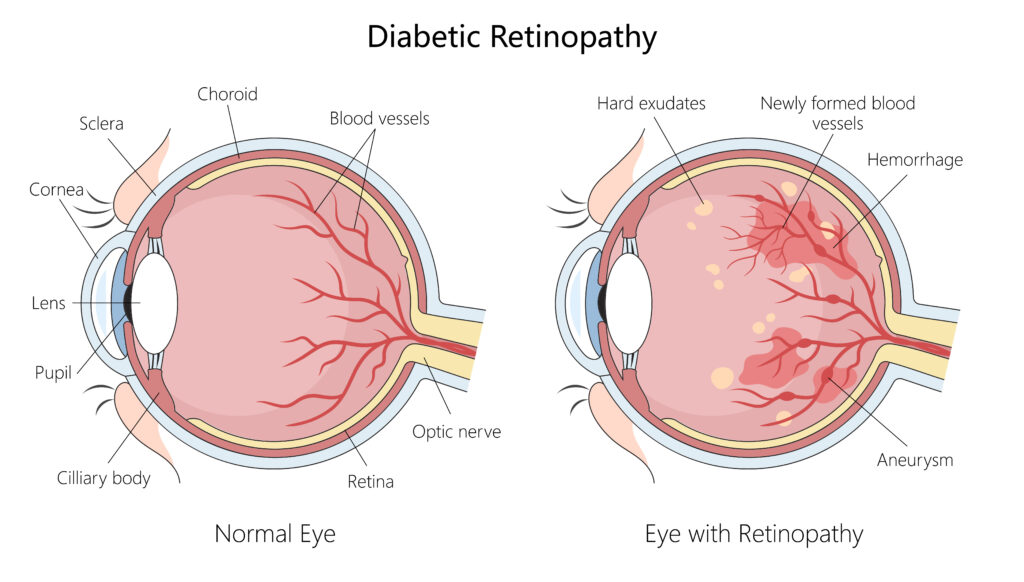
Associated growth of new and fragile capillaries in the retina increases the risk of haemorrhage and loss of vision.22
Chronic hyperglycaemia is higher in Māori and Pacific youth with type 2 than in Europeans,23 and is likely to explain their increased risk of renal and retinal disease,24,25 as there are no differences in rates of hypertension or lipid profiles.8,23
Nephropathy (renal disease): Renal disease is the most common complication for youth with type 2 diabetes. Albuminuria is an early marker of diseased glomerular capillaries and renal disease.
A small amount of albumin detected in the urine, urinary albumin:creatinine ratio (ACR) 3-30mg/mmol, is referred to as microalbuminuria; and large amounts of albuminuria, ACR>30 mg/mmol, as macroalbuminuria.26
Youth with type 2 are four times more likely to develop renal disease than those with type 1 diabetes, despite similar HbA1c levels,19,27 occurring in 55 per cent within 15 years of diagnosis.20 Comparatively, it takes 25 years for the same proportion of adults with type 2 diabetes to develop renal disease.20
An audit in primary care in South Auckland showed higher prevalences for renal disease among Pacific (48 per cent) and Māori (41 per cent) young adults (18-40 years) with type 2 diabetes compared with European (34 per cent).24 Similar prevalences (<40 years) were reported for young adults attending outpatient clinics in Auckland, with 44 per cent having albuminuria at diagnosis (including 12 per cent with macroalbuminuria) and was strongly correlated with developing retinopathy.25
Retinopathy: Although the risk of retinopathy is increased for youth who develop hypertension,28 chronic hyperglycaemia is the main risk factor for retinopathy in this population, indicated by similar rates of retinopathy in both youth with type 1 and type 2 diabetes.27
In the TODAY and SEARCH studies, rates were similar for youth with type 1 and type 2 diabetes with 50-56 per cent having retinopathy within 12 years of diagnosis.28 In Auckland, 56 per cent of young adults with type 2 diabetes had evidence of retinopathy at six years following diagnosis, and the development of retinopathy was associated with duration and glycaemic control.25
Peripheral neuropathy: In addition to chronic hyperglycaemia, central obesity (visceral abdominal fat, indicating insulin resistance), elevated blood pressure and dyslipidaemia all increase the risk of peripheral neuropathy.29
Loss of capillaries serving peripheral nerves affects motor, sensory and autonomic nerves, causing numbness, pain and paraesthesia in hands or feet, and most commonly results in accidental, unnoticed injuries leading to foot disease.
Peripheral neuropathy is more prevalent in youth with type 2 than type 1 diabetes, with 26 per cent, compared to 8 per cent, of each developing peripheral neuropathy after eight years in the SEARCH cohort.19,29 For youth with type 2 in the TODAY cohort, 32 per cent developed peripheral neuropathy within 15 years of diagnosis.20
Peripheral neuropathy is under-reported for this population in New Zealand,25 as is the incidence of all lower leg and foot disease in the TODAY and SEARCH cohorts, suggesting a later onset of neuropathic-associated foot disease.
Macrovascular / cardiovascular complications
Dyslipidaemia (elevated LDL-C and TAGs and reduced HDL-C) and smoking drive atherosclerosis (plaque formation), and, along with hypertension, greatly increase the risk of major cardiovascular events (myocardial infarction, stroke and peripheral vascular events).21,28
Renal disease and retinopathy are also correlated with a higher risk of cardiovascular disease,25 reflecting common risk factors.
In the TODAY cohort, 54 per cent of youth with type 2 diabetes developed >2 cardiovascular risk factors within 14 years of diagnosis, including 59 per cent with hypertension, 33 per cent with elevated LDL-C levels, and 37 per cent with high TAGs. Furthermore, 17 young people (2.5 per cent) had a major cardiovascular event over 17 years.11 Youth of non-European ethnicity also had increased risk of macrovascular complications compared with Europeans.19
In Auckland, 57 per cent of youth with type 2 diabetes had hypertension and 38 per cent and 33 per cent had elevated LDL-C and TAGs, respectively, at the time of diagnosis.23 In the Taranaki region, 43 per cent of obese children and adolescents (including 61 per cent with type 2 diabetes) had dyslipidaemia, and 11 per cent had hypertension or pre-hypertension, while smoking was not reported.8
Smoking increases LDL-C, inflammatory changes in the endothelial layer, increasing atherosclerosis and risk of cardiovascular events.21 Reports are limited on the prevalence of youth with type 2 who smoke but high rates were reported in Australia (38 per cent),27 39 per cent29 and 23 per cent11 in the SEARCH and TODAY cohorts, and for young Māori (43 per cent) and Pacific (24 per cent) adults in Auckland with type 2 diabetes.25

Management
Outcomes are improved by holistic care, family involvement for lifestyle changes, and follow-up from a multi-disciplinary team, including dietitians, psychologists, doctors, and nurses, with clear communication between primary and secondary care services.12
Management recommendations for children and youth with type 2 diabetes in New Zealand are based on Australian,30 American ADA and the UK NICE guidelines.10 Lifestyle and pharmaceutical intervention guidelines are largely based on adult randomised controlled trials and aim to normalise HbA1c to <48 mmol/mol,10 or <53 mmol/mol9 and blood pressure, improve lipid profiles and support smoking cessation.
-
Lifestyle interventions
Recommended lifestyle changes are based on attaining age and developmentally appropriate nutrition,9 60 minutes of moderate to vigorous exercise each day and strength-based exercises three times a week.30 Additional recommendations include limiting recreational screen time to less than two hours a day and ensuring adequate sleep.9
Lifestyle interventions improve cardiometabolic risk profiles (hypertension and dyslipidaemia) and are broadly recommended for those not meeting targets, but improvements decline if nutritional and exercise programmes are not maintained.12,17

Despite recommendations, several activity-based interventions have been tried with limited success in this population in Australasia, including “Push Play NZ” (1999-2002) and the Australian “It’s Your Move” (2006-08), with similar results for lifestyle interventions in other countries. Most youth are unable to achieve or maintain good glycaemic control with lifestyle changes or meet and maintain recommended physical activity levels.17
Smoking: Preventing smoking is ideal and smoking cessation vital for reducing complications in this population.26 Although not reported in New Zealand, it is expected that the proportion of youth with type 2 diabetes who smoke is similar to the 23−39 per cent reported for Australia and North America.11,27,29 Reporting of these rates and evaluation of management strategies would be helpful.
-
Pharmacotherapy
Hyperglycaemia: Until recently, only metformin and insulin were approved for children and youth with type 2 diabetes in New Zealand. Metformin (biguanide) <2 g daily remains the first-line treatment for children10 and people >15 years.26
Metformin reduces insulin resistance, promotes glucose uptake in liver, adipose and skeletal muscle, reduces the breakdown of glycogen and release of glucose and TAGs,26 and improves both microvascular and macrovascular outcomes, highlighted in the first adult metformin UK Prospective Diabetes Study Group (UKPDS-34) trial.
Despite metformin therapy, beta-cell function declined 20-35 per cent each year among youth in the TODAY cohort, (more rapidly than the 7-11 per cent expected for adults) necessitating insulin therapy for half of the cohort in the first year after diagnosis.11
Insulin is recommended in New Zealand if HbA1c is >90 mmol/mol.26 However, insulin, an anabolic hormone, often leads to weight gain, increases episodes of hypoglycaemia17 and stigma, and presents administration challenges for youth.17
Most youth are unable to achieve or maintain good glycaemic control with lifestyle changes or meet and maintain recommended physical activity levels
Second-line therapies now recommended are sodium-glucose cotransporter-2 inhibitors (SGLT2i: empagliflozin), or glucagon-like peptide-1 receptor agonists (GLP-1RA: liraglutide and dulaglutide) and are largely used “off label” as they are not registered in New Zealand for people under 18 years of age.9,26
It is recommended that an SGLT2i or GLP-1RA is added to metformin if HbA1c remains >64 mmol/mol or there is evident renal disease.9,26 Currently in New Zealand, SGLT2i and GLP-1RA have special authority approval. Limited supplies of GLP-1RA and funding by PHARMAC to one agent, however, reduces access for most youth with type 2.26
SGLT2i block glucose transporters in the kidney tubule cells, reducing glucose and water reabsorption, and, in turn, blood volume and blood pressure, reducing the risk of major cardiovascular events in adult trials.31 GLP-1RA mimic the peptide-based incretin hormone released by L-cells in the small intestine, thereby increasing insulin secretion, delaying gastric emptying, reducing glucagon secretion from the alpha cells and hepatic insulin resistance, and decreasing appetite — thus promoting weight loss.31
DDP-4-inhibitors (vildagliptin and linagliptin) inhibit enzymatic degradation of incretins and GLP-1RA, prolonging their glucose-lowering actions. Responses to novel additional hyperglycaemic agents differ between youth and adults, with SGLT2i (empagliflozin), but not linagliptin, slightly reducing HbA1c in youth, and neither drug reducing blood pressure, differing from improvements in cardiovascular risk factors observed in adult trials.31
Vildagliptin can be used as a second-line agent, pioglitazone (a thiazolidinedione that reduces insulin resistance) as a third-line agent, along with sulphonylureas for adults over 18 years, although this may also be considered and recommended by a paediatric endocrinologist for children and adolescents.9,26
Body weight: The TODAY participants trialled a lifestyle-intervention programme compared with metformin and showed a similar reduction in body mass index (BMI) but a greater reduction in HbA1c, opposite to results in adult trials.11
In addition to reducing HbA1c levels, Ozempic (semaglutide), a GLP-1RA, in combination with lifestyle interventions, achieved a 16 per cent reduction in BMI in adolescents, similar to the reduction observed in adults, with mostly transient gastro-intestinal side-effects.32
Longer trials are required to assess maintenance of body weight reductions and potential side effects,32 as continued use of these novel drugs may be required to maintain weight loss.
Bariatric surgery: There are limited studies examining surgical management of youth obesity. One US-based trial reported that most of these youth (89 per cent) lost at least 10 per cent of their total body weight with a mean loss of 27 per cent, and 95 per cent had achieved remission of diabetes and were normoglycemic at three years.33
Improvements in cardiometabolic and renal risk profiles and quality of life were evident for these youth, who had fewer complications than medically-managed youth.33 Down sides of surgery were its association with band slipping or erosion, staple-line leaks, pulmonary embolism, multiple additional surgeries and micronutrient deficiencies.33 Evidence on long-term effects on bone density, malnutrition, mental wellbeing and fertility is lacking.33
Hypertension: The first UKPDS-38 anti-hypertensive trial among adults with type 2 diabetes showed a 37 per cent risk reduction in microvascular complications and a 44 per cent and 32 per cent reduction in stroke and cardiovascular mortality, respectively.
In the TODAY cohort, 19 per cent of youth had hypertension (>130/80 mmHg) at baseline and 59 per cent after 10 years, including 28 per cent prescribed antihypertensive medication.11 The potential benefits of pharmacological management of hypertension in youth with type 2 diabetes are not known.
Dyslipidaemia: It is recommended that LDL-C levels are <1.8 mmol and lipid-lowering therapy (statins) prescribed, if elevated in the presence of cardiovascular risk factors or renal disease.26 One-third of the youth with type 2 develop elevated LDL-C with only 10 per cent prescribed lipid-lowering therapy.11,12
Adding a GLP-1RA (liraglutide) slightly improves lipid profiles and hepatic adiposity, and SGLT2i (empagliflozin) delays initiating insulin.9,31
-
Management challenges
Lifestyle interventions are challenging for this population against a background of urban development and unregulated food-industry commercial interests that perpetuate the obesogenic environment, particularly in economically deprived regions.
A lack of monitoring and under-treatment of hypertension, dyslipidaemia and smoking are evident among youth with type 2 diabetes. There is a paucity of reports on the use and effectiveness of antihypertensive and lipid-lowering medications, and on smoking rates and cessation for this population in New Zealand.
Despite this, 44 per cent of young adults with type 2 diabetes in one Auckland study were prescribed antihypertensives and 38 per cent lipid-lowering medications.25
A lack of monitoring and under-treatment of hypertension, dyslipidaemia and smoking are evident among youth with type 2 diabetes.
Managing hypertension and dyslipidaemia in young women with type 2 diabetes raises safety concerns, given the teratogenicity (risk of causing defects in a developing foetus) of commonly prescribed antihypertensive and lipid-lowering medications. This necessitates caution and, potentially, appropriate contraception.
Conclusion
Youth with type 2 diabetes face an aggressive and treatment-averse condition and experience early and high rates of diabetes-related complications, especially renal disease, within the first and second decades of diagnosis.
Screening and prompt intensive management of hyperglycaemia, hypertension, dyslipidaemia, obesity and smoking are essential to reduce the risk of all diabetes-related complications.
Lifestyle changes and pharmaceutical management pose many challenges for this population, who are more likely to be from economically disadvantaged households.
Family involvement and support, communication between primary and secondary healthcare, and cooperative inter-disciplinary oversight are central to effective management of cardiometabolic risk factors.
Lifestyle changes and pharmaceutical management pose many challenges for this population, who are more likely to be from economically disadvantaged households.
Metformin monotherapy remains the first-line pharmaceutical treatment for managing HbA1c. Most youth with type 2 diabetes require additional glycaemic medications (GLP-1RA or SGLT2i) within one year of diagnosis. Insulin is initiated if HbA1c remains elevated.
More youth intervention and follow-up studies to inform management are required, as current guidelines are largely based on adults who respond differently to some therapies and have a later onset of complications. Central and local government policy changes are urgently required to reduce the current obesogenic environment driving this epidemic among children and youth.
Elisa Wong, RN, MN, is a clinical nurse specialist at Te Toka Tumai / Auckland City Hospital.
Priya Joseph, RN, MN, is a clinical nurse specialist and diabetes RN prescriber at Health New Zealand Te Whatu Ora Waikato.
Catherine Bacon, BPhEd (Hons), MSc, PhD, is a senior lecturer at the School of Nursing, Faculty of Medical and Health Sciences, University of Auckland
Barbara M Daly, RN, PhD, is a senior lecturer in the School of Nursing, Faculty of Medical and Health Sciences, University of Auckland. She can be contacted at [email protected]
* This article was reviewed by Jenny Rayns and Carla Frewen, diabetes clinical nurse specialists in the Te Whatu Ora southern region.
References
- Perng, W., Conway, R., Mayer-Davis, E., & Dabalea, D. (2023). Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care, 46(3), 490-99.
- Haynes, A., Kalic, R., Cooper, M., Hewitt, J. K., & Davis, E. A. (2016). Increasing incidence of type 2 diabetes in Indigenous and non-Indigenous children in Western Australia. Medical Journal of Australia, 204(8), 303.
- Sjardin, N., Reed, P., Albert, B., Mouat, F., Carter, P. J., Hofman, P., Cutfield, W., Gunn, A., & Jefferies, C. (2018). Increasing incidence of type 2 diabetes in New Zealand children <15 years of age in a regional-based diabetes service, Auckland, New Zealand. Journal of Paediatrics and Child Health, 54(9), 1005-1010.
- Valaiyapathi, B., Gower, B., & Ashraf, A. P. (2020). Pathophysiology of Type 2 Diabetes in Children and Adolescents. Current Diabetes Reviews, 16(3), 220-229.
- Pillay, D., Ali, A., & Wham, C. A. (2022). Examining the New Zealand school food environment: what needs to change? Nutrition Research Reviews, 36(2), 406-419.
- Mackay, S., Gerritsen, S., Sing, F., Vandevijvere, S., & Swinburn, B. (2022). Implementing healthy food environment policies in New Zealand: nine years of inaction. Health Research Policy and Systems, 20, 8.
- Magliano, D. J., Sacre, J. W., Harding, J. L., Gregg, E. W., Zimmet, P. Z., & Shaw, J. E. (2020). Young-onset type 2 diabetes mellitus – implications for morbidity and mortality. Nature Reviews Endocrinology, 16(6), 321-331.
- Anderson, Y. C., Wynter, L. E., Treves, K. F., & Grant, C. C. (2016). Prevalence of comorbidities in obese New Zealand children and adolescents at enrolment in a community-based obesity programme. Journal of Paediatrics and Child Health, 52(12).
- Best Practice Advocacy Centre New Zealand. (2022). A rising tide of type 2 diabetes in younger people: what can primary care do? Best Practice Journal.
- National Institute for Health and Care Excellence. (2023). Diabetes (type 1 and 2) in children and young people: diagnosis and management — NICE guideline [NG18] .
- Today Study Group, Shah, R. D., Braffett, B. H., Tryggestad, J. B., Hughan, K. S., Dhaliwal, R., Nadeau, K. J., Levitt Katz, L. E., & Gidding, S. S. (2022). Cardiovascular risk factor progression in adolescents and young adults with youth-onset type 2 diabetes. Journal of Diabetes and its Complications, 36(3), 108123.
- Viner, R., White, B., & Christie, D. (2017). Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet, 389(10085), 2252-2260.
- Kelsey, M. M., & Zeitler, P. S. (2016). Insulin Resistance of Puberty. Current Diabetes Reports, 16(7), 64.
- Kemp, B. J., Parrish, A. M., Batterham, M., & Cliff, D. P. (2020). Participation in Domains of Physical Activity Among Australian Youth During the Transition From Childhood to Adolescence: A Longitudinal Study. Journal of Physical Activity & Health, 17(3), 278-86.
- Mandic, S., Khan, A., Garcia Bengoechea, E., Coppell, K. J., Spence, J. C., & Smith, M. (2024). Physical activity, screen time and dietary behaviours in New Zealand adolescents prior to and following the onset of the COVID-19 pandemic. BMC Public Health, 24(1), 188.
- Dwyer, T., Magnussen, C. G., Schmidt, M. D., Ukoumunne, O. C., Ponsonby, A.-L., Raitakari, O. T., Zimmet, P. Z., Blair, S. W., Thomson, R., Cleland, V. J., & Venn, A. (2009). Decline in physical fitness from childhood to adulthood associated with increased obesity and insulin resistance in adults. Diabetes Care, 32(4), 683-687.
- Lascar, N., Brown, J., Pattison, H., Barnett, A. H., Bailey, C. J., & Bellary, S. (2018). Type 2 diabetes in adolescents and young adults. Lancet Diabetes & Endocrinology, 6(1), 69-80.
- McVoy, M., Hardin, H., Fulchiero, E., Caforio, K., Briggs, F., Neudecker, M., & Sajatovic, M. (2023). Mental health comorbidity and youth onset type 2 diabetes: A systematic review of the literature. International Journal of Psychiatry in Medicine, 58(1), 37-55.
- Dabelea, D., Stafford, J. M., Mayer-Davis, E. J., D’Agostino Jr, R., Dolan, L. Imperatore, G., Linder, B., Lawrence, J. M., Marcovina, S. M., Mottl, A. K., Black, M. H., Pop-Busui, R., Saydah, S., Hamman, R. F., Pihoker, C., SEARCH for Diabetes in Youth Research Group. (2017). Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA, 317(8), 825-835.
- Today Study Group, Bjornstad, P., Drews, K. L., Caprio, S., Gubitosi-Klug, R., Nathan, D. M., Tesfaldet, B., Tryggestad, J., White, N. H., & Zeitler, P. (2021). Long-Term Complications in Youth-Onset Type 2 Diabetes. New England Journal of Medicine, 385(5), 416-426.
- Al Rifai, M., DeFilippis, A. P., McEvoy, J. W., Hall, M. E., (2017). The relationship between smoking intensity and subclinical cardiovascular injury: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis, 258, 119-130.
- Sarmah, S., & Roy, A. S. (2022). A review on prevention of glycation of proteins: Potential therapeutic substances to mitigate the severity of diabetes complications. International Journal of Biological Macromolecules, 195, 565-588.
- Ludwig, K., Craig, M. E., Donaghue, K. C., Maguire, A., Benitez-Aguirre, P. Z., & ADDN Study Group. (2021). Type 2 diabetes in children and adolescents across Australia and New Zealand: A 6-year audit from The Australasian Diabetes Data Network (ADDN). Pediatric Diabetes, 22(3), 380-387.
- Perera, K., Baker, J., Jayanatha, K., Pickering, K., Cutfield, R., Orr-Walker, B., Sundborn, G., Heroy, A., Arnold, T., Yu, D., & Simmons, D. (2024). Risk of renal disease progression in young adults with type-2-diabetes. New Zealand Society for the Study of Diabetes programme handbook 2024, p22.
- Beig, J., Khanolkar, M., & Cundy, T. (2018). Type 2 diabetes in young adults in Central Auckland: demography and complications. Internal Medicine Journal, 48(1), 67-73.
- New Zealand Society for the Study of Diabetes (NZSSD). (2023). Type 2 Diabetes Management Guidance 2023 .
- Constantino, M. I., Molyneaux, L., Limacher-Gisler, F., Al-Saeed, A., Luo, C., Wu, T., Twigg, S. M., Yue, D. K., & Wong, J. (2013). Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care, 36(12), 3863-9.
- Jensen, E. T., Rigdon, J., Rezaei, K. A., (2023). Prevalence, Progression, and Modifiable Risk Factors for Diabetic Retinopathy in Youth and Young Adults With Youth-Onset Type 1 and Type 2 Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care, 46(6), 1252-1260.
- Jaiswal, M., Lauer, A., Martin, C. L., (2013). Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care, 36(12), 3903-8.
- Pena, A. S., Curran, J. A., Fuery, M., George, C., Jefferies, C. A., Lobley, K., Ludwig, K., Maguire, A. M., Papadimos, E., Peters, A., Sellars, F., Speight, J., Titmuss. A., Wilson, D., Wong, J., Worth, C., & Dahiya, R. (2020). Screening, assessment and management of type 2 diabetes mellitus in children and adolescents: Australasian Paediatric Endocrine Group guidelines. Medical Journal of Australia, 213(1), 30-43.
- Laffel, L. M., Danne, T., Klingensmith, G.J., (2023). Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes & Endocrinology, 11(3), 169-181.
- Weghuber, D., Barrett, T., Barrientos-Perez, M., Gies, I., Hesse, D., Jeppesen, O. K., Kelly, A. S., Mastrandrea, L. D., Sørrig, R., & Arslanian, S., for the STEP TEENS Investigators. (2022). Once-Weekly Semaglutide in Adolescents with Obesity. New England Journal of Medicine, 387(24), 2245-57.
- Inge, T. H., Laffel, L. M., Jenkins, T. M., (2018). Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatrics, 172(5), 452-460.





