KEY POINTS
- A biosimilar medicine, Amgevita, is set to replace the reference biological medicine, Humira, as the main funded adalimumab option.
- From October 1, 2022, Humira will remain available to existing patients only under specific circumstances.
- Health professionals’ conversations with patients about changing from Humira to Amgevita should be initiated early and framed positively; stable patients may be transitioned in primary care.
- Patients will need training on how to inject Amgevita, as the devices differ from those used to administer Humira.
- To avoid inadvertent substitution, always use brand names when dealing with biological medicines.
Adalimumab is a biological medicine taken by thousands of New Zealanders to treat autoimmune disorders. Next month Pharmac is shifting its adalimumab funding to a cheaper biosimilar version of the drug called Amgevita.
This article summarises the changes to adalimumab funding and access. It also provides background and resources to foster confidence in biosimilars, and outlines the supports needed to change patients from Humira to Amgevita.
Introduction
Biological medicines have markedly improved the prognoses for many chronic and disabling inflammatory and immunological conditions and cancers. Their use in New Zealand is increasing and, as patents expire on the original or “reference” biological medicines, competitor manufacturers can produce very similar versions, called biosimilars, at a fraction of the cost.1,2
On March 1 this year, the reference adalimumab medicine, called Humira, ceased being funded for new patients. All new patients starting adalimumab now receive a biosimilar version, called Amgevita, and this will become the primary funded option for adalimumab from October 1.3
This means that, with a few exceptions, patients currently on Humira (ie those who were on it before March 1) will need to be changed to Amgevita by October 1 to continue receiving funded treatment. Many patients who are stable on Humira already have repeat prescriptions and Special Authority renewals actioned in primary care. Pharmac is encouraging general practice teams to transition these patients to Amgevita.3
What is adalimumab?
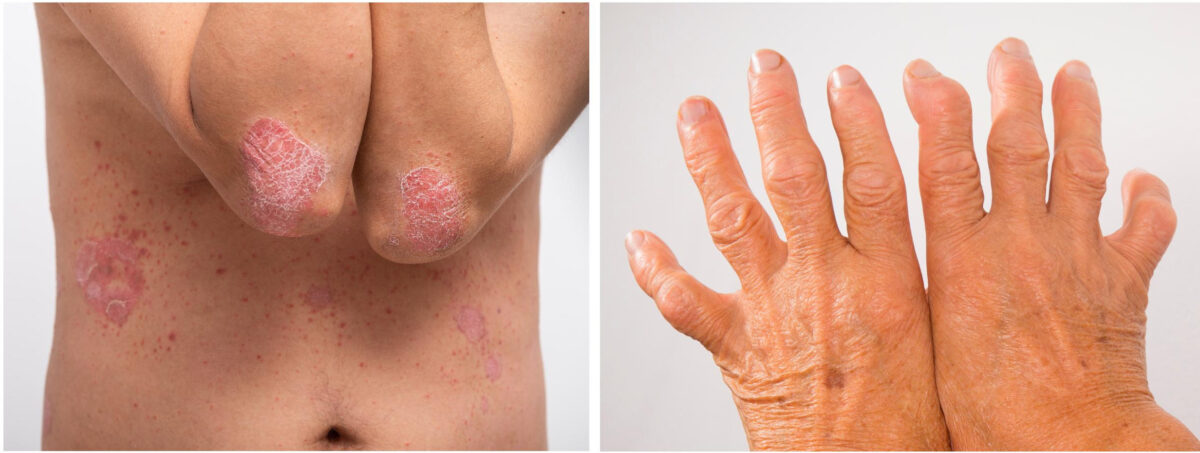
Adalimumab is a human anti-tumour necrosis factor alpha (TNF-α inhibitor) monoclonal antibody that blocks inflammatory and immune responses often associated with chronic autoimmune conditions. It is used to treat a range of dermatological, rheumatological, gastrointestinal and ophthalmologic conditions.4
The reference adalimumab, Humira, has been on the New Zealand Pharmaceutical Schedule since 2009 and funded under Special Authority criteria for use in:5,6
-
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- psoriatic arthritis
- ankylosing spondylitis
- Crohn disease
- plaque psoriasis
- hidradenitis suppurativa
- uveitis (ocular inflammation).
About 6400 New Zealand patients routinely self-administer adalimumab fortnightly by subcutaneous injection. Of those who are seen in primary care, most have either rheumatoid or inflammatory bowel conditions.7
The biosimilar agent Amgevita costs much less than the reference biological, Humira ($375 vs $1600 per month for adults). This saving has allowed Pharmac to widen the access criteria (see Panel 1), and it is anticipated that 700 more people will have access to adalimumab in the first year.3
Safety and efficacy of biosimilar medicines
Adalimumab biosimilars have been shown internationally to be equally effective to the reference adalimumab product. There is no evidence to indicate any specific clinical risk or harm with switching from the reference product to a biosimilar.8 On reviewing the literature, New Zealand’s Pharmacology and Therapeutics Advisory Committee (PTAC) concluded there was currently no evidence that the rate of development of immunogenicity – including the identification of treatment antibodies leading to loss of treatment effectiveness – differed between reference and biosimilar adalimumab.8
There is no evidence to indicate any specific clinical risk or harm with switching from the reference product to a biosimilar.
Amgevita has been approved by the European Medicines Agency (EMA) for use in the European Union (EU), by the Food and Drug Administration in the United States, by the Therapeutic Goods Administration in Australia, and by Medsafe in New Zealand for all the indications for which Humira is approved.
More than 15 years’ use of biosimilars in the EU, over two billion treatment days worldwide, and reviews of more than 175 reference/biosimilar switch studies conducted up to 2018 have not revealed any safety problems.9
Timeline for changing from Humira to Amgevita
 All existing Humira patients were issued a Special Authority number for Amgevita in March, and a seven-month transition window was put in place (to October 1, 2022).3
All existing Humira patients were issued a Special Authority number for Amgevita in March, and a seven-month transition window was put in place (to October 1, 2022).3
From March 1 to September 30, 2022
- Amgevita or Humira are both funded for existing patients and uses. (All prescriptions must clearly specify the brand of adalimumab — Amgevita or Humira — required.)
- Most patients using Humira should be changed to Amgevita (see exceptions below).
- Only Amgevita will be funded for new patients and uses.
From October 1, 2022
-
- Amgevita will be the main funded brand of adalimumab for all uses (current and new).
- Humira will remain funded (through a new initial Special Authority – see below) for patients previously treated with Humira who, following discussion with their prescriber:
-
- have Crohn disease or ocular inflammation and are considered at risk of disease destabilisation if there were to be any change to their treatment regimen.
- trial at least two doses of Amgevita (for less than six months after starting Amgevita) and experience clinical difficulties (intolerable side effects or loss of disease control attributed to the change) and choose to return to Humira.
-
- All existing Special Authorities for Humira will expire on September 30, 2022, and the appropriate prescriber will need to complete a new initial Special Authority for the above patients who require ongoing funded access to Humira.
Note that the Special Authorities for Humira and Amgevita are not interchangeable.
Panel 1: Expanded access to adalimumab via prescribing of Amgevita10
New funded indications from March 1, 2022, include:
- ulcerative colitis first-line
- undifferentiated spondyloarthritis
- inflammatory bowel disease-associated arthritis.
The following currently funded indications also have access widened from March 1, 2022:
- Crohn disease dose escalation
- rheumatoid arthritis: reduction in the number of swollen joints required for access to treatment, and removal of the requirement for C-reactive protein level to be >15mg/L
- Behçet disease; access to funded treatment with adalimumab as a first-line biologic
- ocular inflammation; access to funded treatment with adalimumab as a first-line biologic.
Funded access to adalumimab for all indications will be improved by the new Special Authority criteria for Amgevita. These will include:
- removal of dosage restrictions
- extension of Special Authority renewal periods to two years
- allowing any relevant practitioner to apply for Special Authority renewals
- removal of Special Authority renewals for some conditions.
Primary health care providers will be able to alert adalimumab-naive patients to the expanded access criteria and, where appropriate, refer for secondary care consultation for potential initiation of the medicine.
The full Amgevita Special Authority criteria are available in the Pharmaceutical Schedule.
Transitioning patients from Humira to Amgevita
 Based on overseas experience, patient transition from Humira to Amgevita in primary care does not present difficulties, provided the person is given adequate support and information about the change.
Based on overseas experience, patient transition from Humira to Amgevita in primary care does not present difficulties, provided the person is given adequate support and information about the change.
Patients using Humira should only receive a first dispensing of Amgevita after having a supporting appointment or discussion with an appropriate prescriber. Other primary health care professionals involved in the care of patients using Humira should initiate a conversation about transitioning to Amgevita at the earliest opportunity.
Transitioning stable patients from Humira to Amgevita will benefit from a multi-stakeholder approach by specialists, GPs, primary care nurses and pharmacists; good communication between primary and secondary care; and knowledgeable and positive conversations with the patient about biosimilars and Amgevita.
The role of each health-care professional is to ensure that patients receive the right treatment and support. The changes to adalimumab funding provide additional flexibility in primary care management of patients while maintaining engagement with secondary care as needed.
Everyone in the health system has a role to play to deliver this change, and some specific roles may include:
Specialists: New patients (adalimumab-naive) and patients accessing Amgevita under new or expanded existing indications will continue to have treatment initiated by a specialist. During the transition of stable patients from Humira to Amgevita, specialist support may be sought for:
-
-
-
- patients with additional needs and/or unstable disease
- patients whose disease control has become reduced during the transition
- patients for whom the primary health care professional does not feel confident in managing the transition.
-
-
General practitioners, nurse practitioners, nurse prescribers, pharmacist prescribers: Stable patients transitioning from Humira to Amgevita may be managed in primary care, which can take responsibility for:
-
-
-
- Amgevita Special Authority renewals (by any relevant practitioner) – renewals will only be required every two years, reducing administrative burden
- ongoing prescribing of adalimumab in the community
- the referral of patients to relevant specialists where appropriate (eg, additional needs, evidence of disease deterioration)
- helping patients feel confident and comfortable using Amgevita
- potentially, some training of patients about how to use the Amgevita device
- monitoring for treatment efficacy and adverse effects.
-
-
Primary care nurses: Nurses in primary care will have an important role in:
-
-
- training patients in the use of the Amgevita device (previously a role performed by secondary care nurses)
- ensuring patients feel confident and comfortable using Amgevita
- monitoring for treatment efficacy and adverse effects.
-
Community pharmacists: Patients presenting prescriptions for Humira should be given information about the funding changes and prompted to make an appointment to discuss the transition to Amgevita with the appropriate prescriber. The pharmacist has the opportunity also to:
-
-
-
- support the training of patients on how to use the Amgevita device
- ensure patients have received appropriate training at first dispensing of Amgevita
- provide information to promote confidence in Amgevita at first dispensing.
-
-
Community pharmacists can initiate the conversation as Humira prescriptions are filled, recommending the patient talk with their prescriber.
See Panel 2 for resources to help with the transition.
Panel 2: Amgevita resources
Resources for primary health care providers
To support the introduction of Amgevita, resource packs for prescribers, nurses and pharmacists are available from the supplier, Amgen, including:
- clinical information about Amgevita, including efficacy, safety and immunogenicity data
- practical information about Amgevita, including use of the device and patient training
- reusable demonstration devices and links to online learning modules
- access to a free medical information phone line to support health-care professionals with logistical, practical and clinical queries.
Resources for patients
Patients who are prescribed Amgevita will have the following information and support from Amgen:
- key information about Amgevita, device instructions, a patient alert card and information about how to access support services, in a range of languages
- free access to registered nurses (based in Australia) via phone and video to assist with self-administration and medicine queries
- free replacement sharps bins posted directly to patients upon request
- a website with access to supporting documents and videos, and links for ordering sharps bins and contacting remote nursing support.
Links to additional resources can be found at the He Ako Hiringa “Biological medicines resource hub”.
Amgevita product characteristics
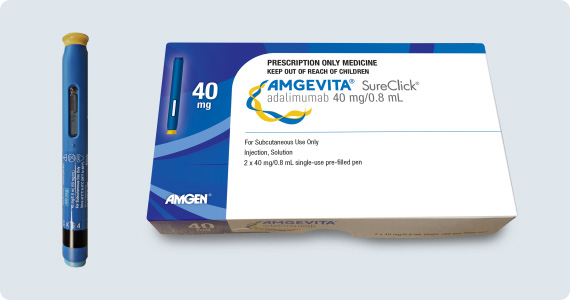
Amgevita is supplied as a citrate-free formulation in a 20mg (paediatric) dose pre-filled glass syringe and as a 40mg dose, in either a pre-filled glass syringe or pre-filled pen.4,11 The Amgevita pen is similar to the Humira device but differs in shape and colour. Both use a clear window that fills yellow over 10 seconds as the injection is delivered.
-
-
- Before subcutaneous administration, the Amgevita device should be allowed to rest at room temperature for 15-30 minutes. It should not be warmed in any other way.4
- The solution should be inspected closely and not used if it is discoloured, cloudy, or if flakes or particles are present. Vigorous shaking of the product is to be avoided.4
- Amgevita does not contain preservatives; any unused medicine or waste material should be disposed of appropriately.4
- The longer shelf life of Amgevita (36 months vs 24 months for Humira) may be advantageous for pharmacists in terms of holding stock.
- Amgevita is available in the same dose and delivery options in which Humira has been available – see Table 1.3
-
Amgevita product information can be found on the Amgen website.
Table 1. Amgevita doses and delivery
| Chemical | Formulation | Brand | Pack size |
|---|---|---|---|
| Adalimumab | Inj 20mg per 0.4ml prefilled syringe | Amgevita | 1 |
| Adalimumab | Inj 40mg per 0.8ml prefilled syringe | Amgevita | 2 |
| Adalimumab | Inj 40mg per 0.8ml prefilled pen | Amgevita | 2 |
Using brand names
In all professional and patient interactions, biological medicines should be referred to by their brand name. The New Zealand Formulary recommends to:
Reporting adverse events
If a patient has a suspected adverse drug reaction to a biological medicine, a report should be submitted to the Centre for Adverse Reactions Monitoring (CARM) at the New Zealand Pharmacovigilance Centre. All reports should include the brand name and batch number of the suspected biological medicine and can be made via the website, by email ([email protected]) or by using a pre-printed card. Electronic reporting is also possible using an adverse reaction reporting tool present in many practice management systems.1
Patient education and positive conversations
Successful transition of patients from Humira to Amgevita requires interdisciplinary cooperation and consistent messaging by specialists, primary care prescribers, nurses and pharmacists. As an exercise involving biosimilars, it is one that will become increasingly relevant for health-care professionals as more products come onto the market.
A smooth transition for the patient depends on health-care professionals:
-
-
- being a trusted source of knowledge on biologics and biosimilars
- explaining the reasons for, and benefits of, the change
- managing patient anxiety about change
- having positive conversations with patients about Amgevita and promoting expectations of continued disease control
- providing device training.
-
Good conversations with patients before initiating or changing a medicine help to reduce the likelihood of unwanted outcomes due to the nocebo effect.14 The nocebo effect is a decrease in subjective benefit, a worsening of symptoms or onset of adverse effects due to a patient’s expectation or perception of harm associated with a treatment.14

Patient reporting of adverse drug effects is very much related to their expectations and may be affected by how the health-care professional talks about the medicine. In general, people are much more inclined to pick up on negative rather than positive information, both from outside and online sources, and any hesitancy or doubt conveyed to them by health professionals.15
A practitioner who is hesitant, diffident or uncertain can transfer these feelings during the consultation, affecting how the patient experiences and accepts a medicine.
Health-care professionals are instrumental in framing medicines positively or negatively and their own beliefs can significantly influence how a patient feels about a medicine. A practitioner who is hesitant, diffident or uncertain can transfer these feelings during the consultation, affecting how the patient experiences and accepts a medicine.15,16
A useful approach is to create an environment where the patient can voice their beliefs, and for the prescriber to provide information – perhaps using a health literacy framework to aid the conversation:17
-
-
- Ask the patient how they feel about changing from Humira to Amgevita.
- Build on their existing knowledge of the medicines.
- Check with the patient that you have explained things adequately and they know the next steps to be taken.
-
He Ako Hiringa has produced a bulletin and podcasts about positive framing when initiating new medicines.
Panel 3: Terminology is important
When using the terms switching, transitioning and substitution, it pays to be clear.
SWITCHING is when the treating clinician acts “to exchange one medicine for another with the same therapeutic intent”.13 Switching can refer to a change between two different medicines (eg, infliximab to adalimumab) or between a reference biological medicine and its biosimilar (eg, Humira to Amgevita) or between biosimilars of the same reference product.
Switching from a reference product to a biosimilar (or vice versa) or between biosimilars is also referred to as nonmedical switching (ie, for cost-saving purposes18 – it has been proposed the term TRANSITIONING is used for this type of switching to help delineate the types of switches reported in the literature.18,19
AUTOMATIC SUBSTITUTION is where a medicine is dispensed in place of another equivalent medicine that is expected to have the same clinical effect, at the pharmacy level without consultation with the prescriber.13 Note that, in New Zealand, automatic substitution of biological medicines is not permitted.20
Reading this article, and following the links to the bulletin and podcasts can equate to one hour of CPD time.
Nurses can use the Nursing Council’s professional development activities template to record professional development completed via Kaitiaki, and they can then have this verified by their employer, manager or nurse educator.
References
-
- bpacnz. (2020). Biosimilars: the future of prescribing biological medicines.
- He Ako Hiringa. (2021). Biosimilars: A promising new era. Bulletin 7.
- Pharmac. (2021). Decision to widen access to adalimumab and award Principal Supply.
- Medsafe. (2022). New Zealand data sheet: Amgevita.
- Pharmac. (2021, August 26). Proposal to widen access to adalimumab and award Principal Supply.
- Pharmac. (2021). Adalimumab (Humira): Alternative brand access.
- Pharmac. (2021, March 9). Request for Proposals – Supply of Adalimumab.
- Pharmac. (2020). Record of the New Zealand Pharmacology and Therapeutics Advisory Committee meeting, November 12-13.
- Kurki, P., Barry, S., Bourges, I., Tsantili, P., & Wolff-Holz, E. (2021). Safety, immunogenicity and interchangeability of biosimilar monoclonal antibodies and fusion proteins: A regulatory perspective. Drugs, 81(16), 1881-96. https://doi.org/10.1007/s40265-021-01601-2
- Pharmac. (2022). Adalimumab (Amgevita): Information for health care professionals.
- Amgen (New Zealand) Limited. (2022). What’s behind Amgevita makes the difference.
- New Zealand Formulary. Guidance on medicines use: Biological and biosimilar medicines.
- The European Medicines Agency and the European Commission. (2019). Biosimilars in the EU: Information guide for healthcare professionals.
- bpacnz. (2019). The nocebo effect: what is it, why is it important and how can it be reduced?
- Petrie, K. (2021). Legendary Conversations (podcast). Episode One: Initiating new medicines (part 1). He Ako Hiringa.
- He Ako Hiringa. (2021). Starting a medicine? Accentuate the positive. Bulletin 9.
- Health Quality & Safety Commission New Zealand. (2021). Three steps to better health literacy – a guide for health care professionals.
- Barbier, L., Ebbers, H. C., Declerck, P., Simoens, S., Vulto, A. G., & Huys, I. (2020). The efficacy, safety, and immunogenicity of switching between reference biopharmaceuticals and biosimilars: A systematic review. Clinical Pharmacology & Therapeutics, 2020;108(4):734-55.
- Dörner, T., & Kay, J. (2015). Biosimilars in rheumatology: current perspectives and lessons learnt. Nature Reviews Rheumatology, 11, 713-724.
- Medsafe. (2014). Biosimilars.